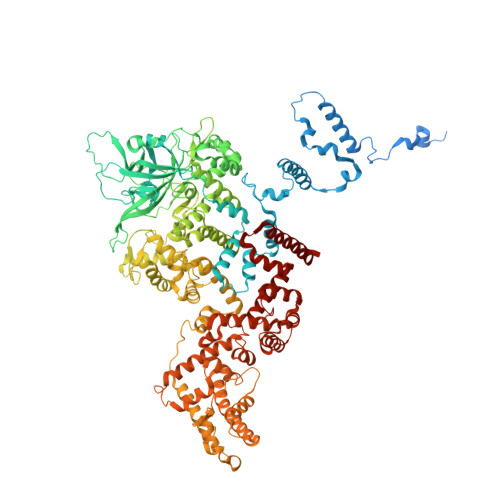
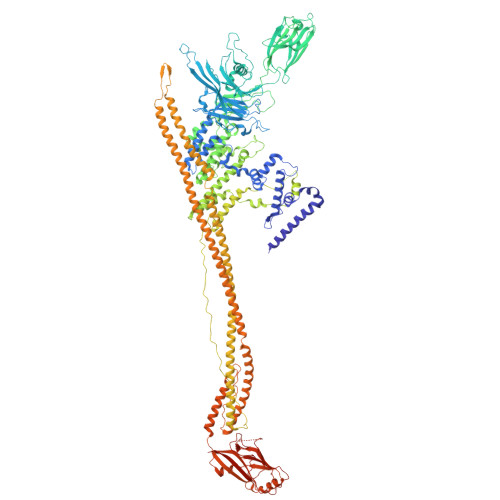
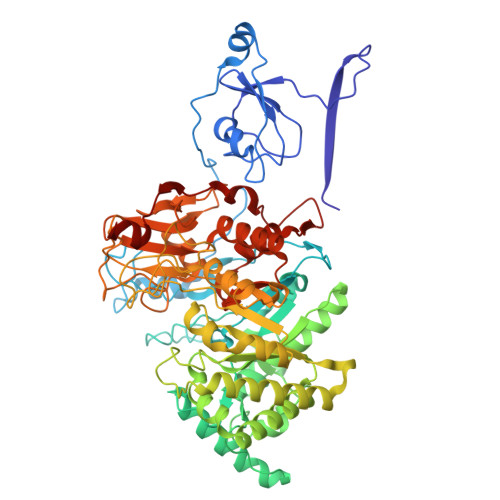
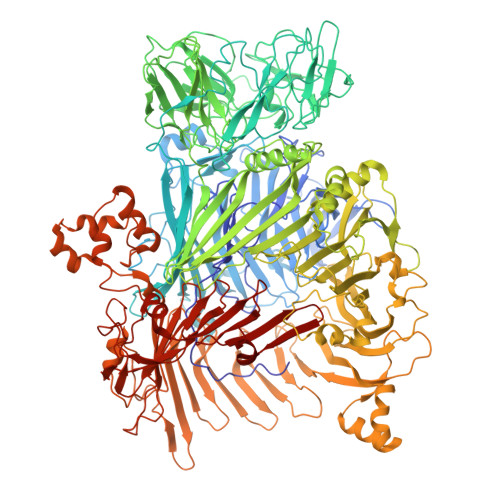
Complete structures of the YenTc holotoxin prepore and pore reveal the evolutionary basis for chitinase incorporation into ABC toxins.
Low, Y.S., Roche, S.G., Aleksandrova, N.A., Foley, G., Low, J.K., Box, J.K., Croll, T.I., Chassagnon, I.R., Lott, J.S., Deplazes, E., Boden, M., Hurst, M.R., Piper, S.J., Landsberg, M.J.(2025) Nat Commun 16: 11121-11121
- PubMed: 41397958
- DOI: https://doi.org/10.1038/s41467-025-66050-x
- Primary Citation of Related Structures:
9C4K, 9CBC - PubMed Abstract:
ABC toxins are toxin-translocating, pore-forming proteins found in a wide range of insecticidal bacteria and some mammalian pathogens. The Yersinia entomopahaga toxin complex (YenTc) belongs to a distinct subclass of ABC toxins, defined by a divergent molecular architecture. Structural details that define their mechanism of action remain to be elucidated. Here we determine structures of the YenTc holotoxin assembly in both prepore and pore-forming configurations using cryo-EM in conjunction with Alphafold2-assisted structural modelling of flexible domains. We define the structural mechanism via which enzymatically-active chitinase subunits are incorporated, and show using phylogenetic analyses that this subclass-defining feature has evolved relatively recently. Our structures point to the existence of distinct conformational states in YenTc, which may distinguish it from other structurally-characterised ABC toxins, or represent states on a shared mechanistic trajectory. Thus, our findings enhance our understanding of the structural diversity that defines distinct ABC toxin subclasses.
- School of Chemistry and Molecular Biosciences, The University of Queensland, St Lucia, QLD, Australia.
Organizational Affiliation: