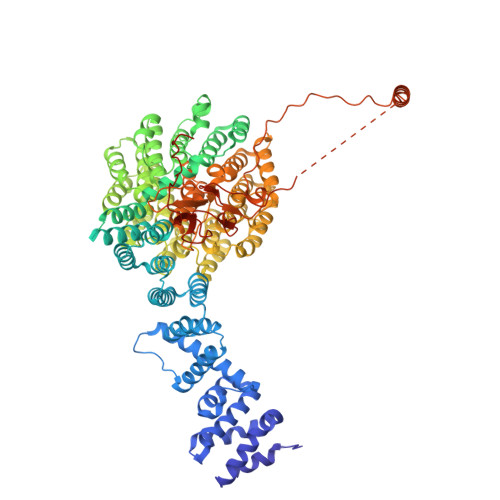
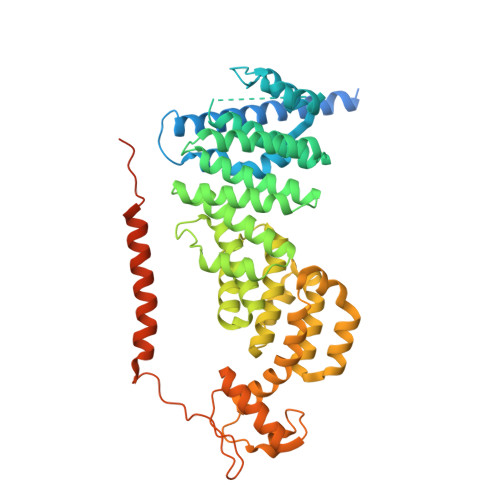
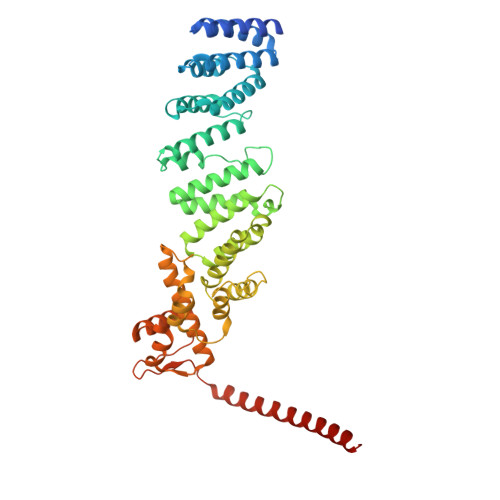
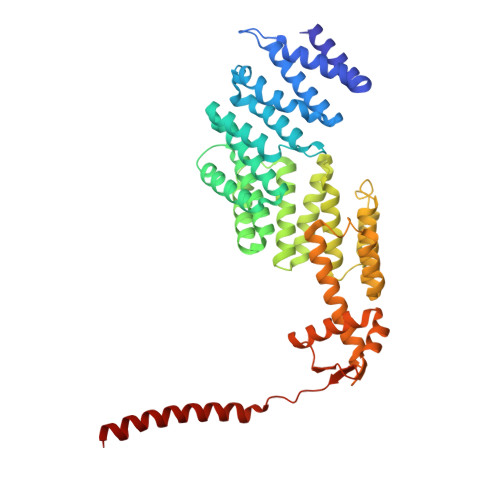
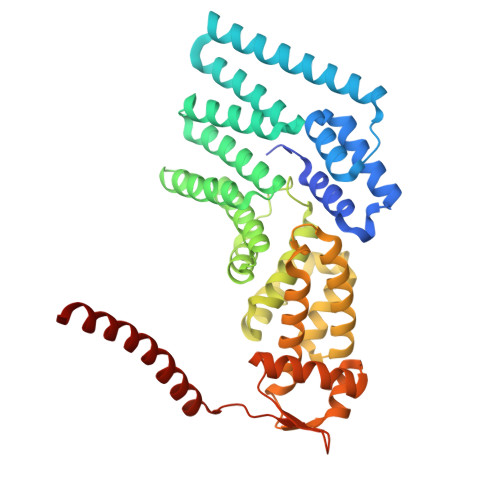
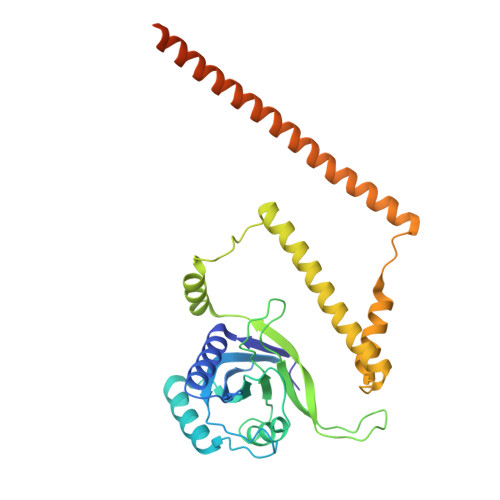
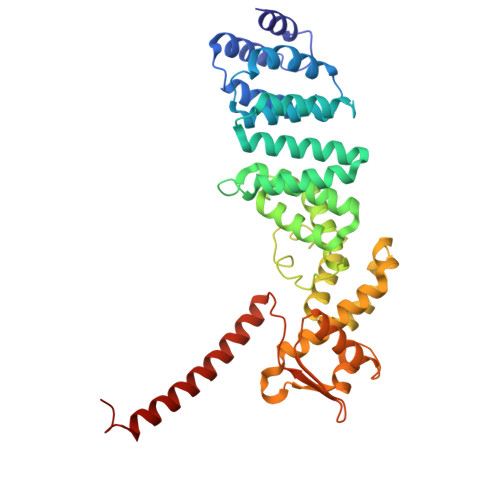
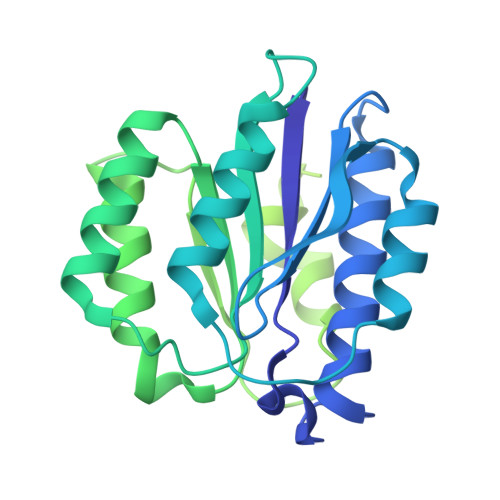
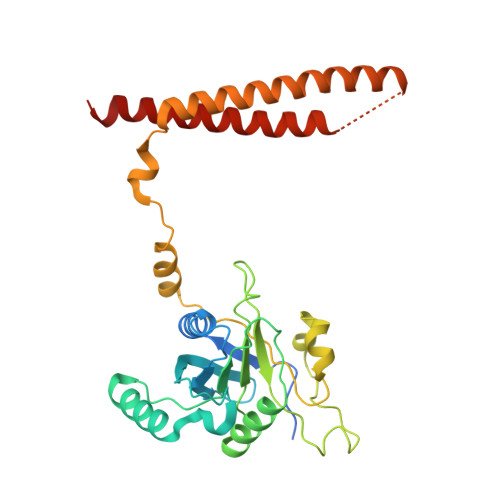
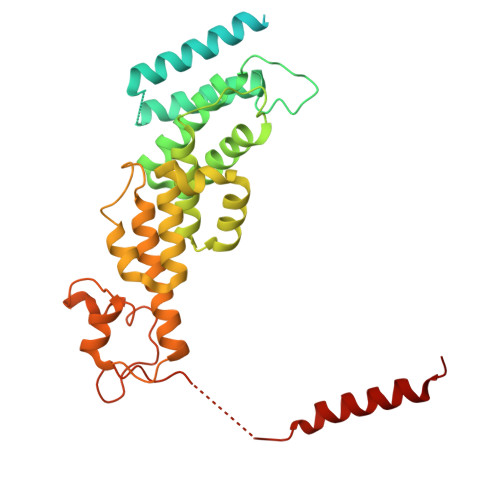
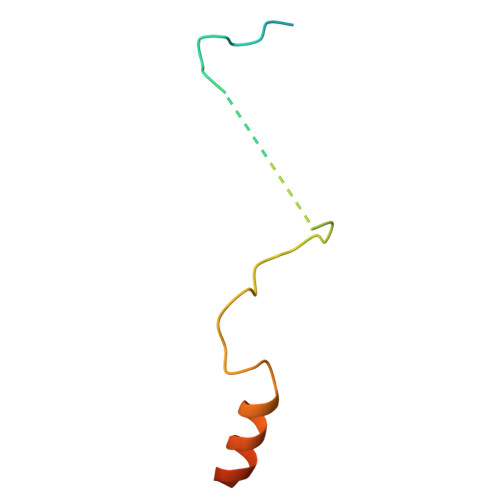
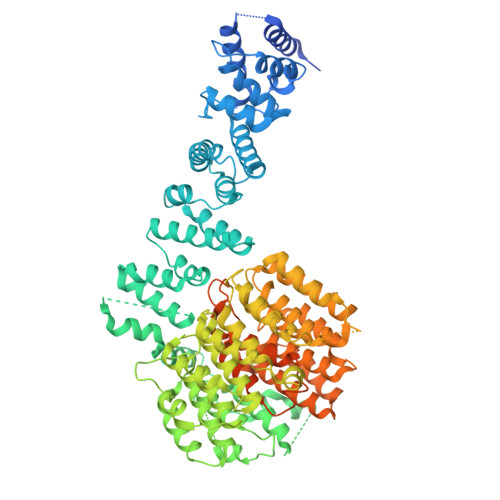
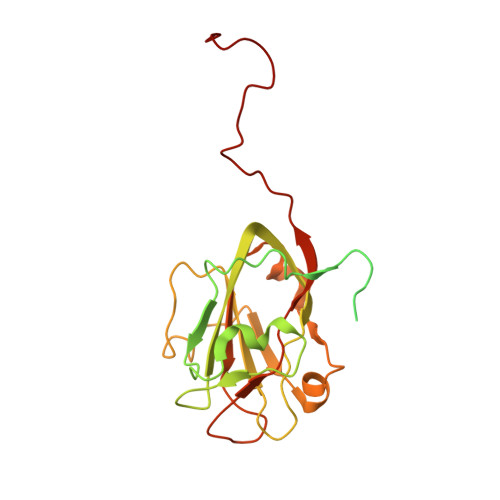
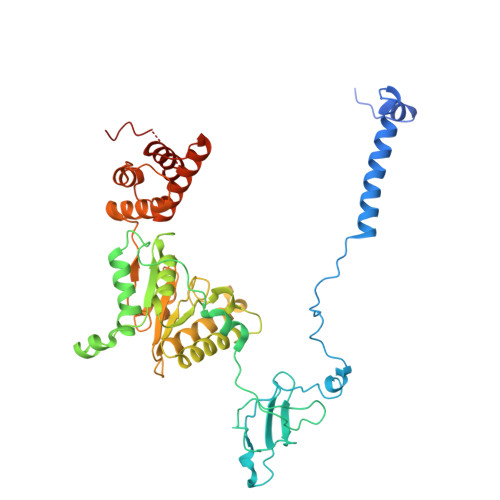
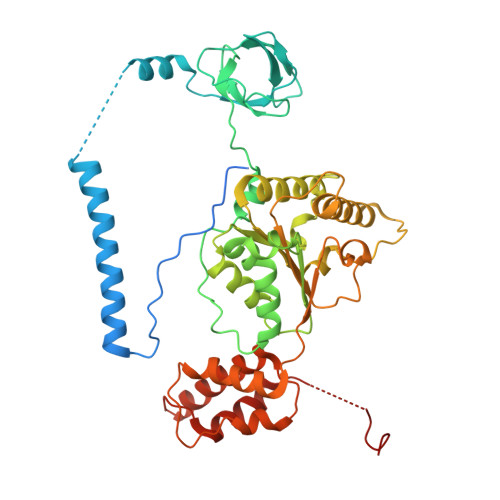
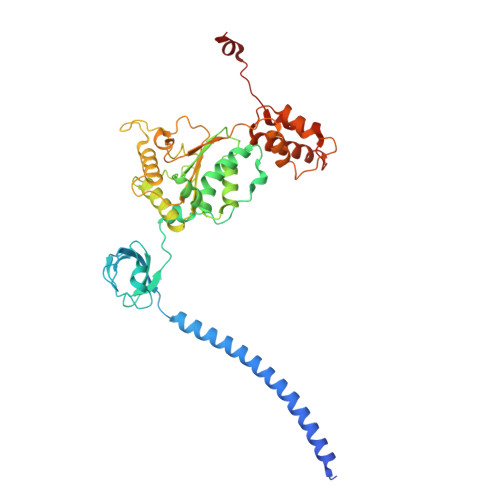
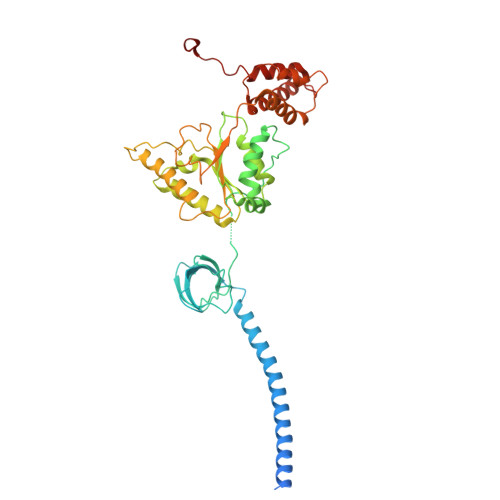
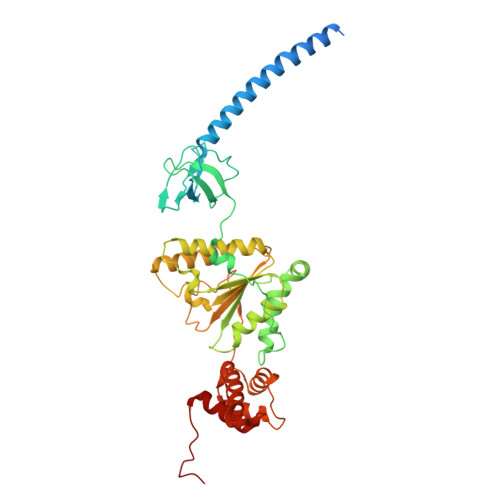
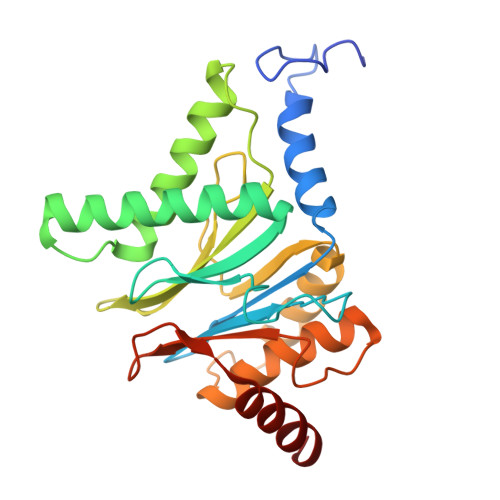
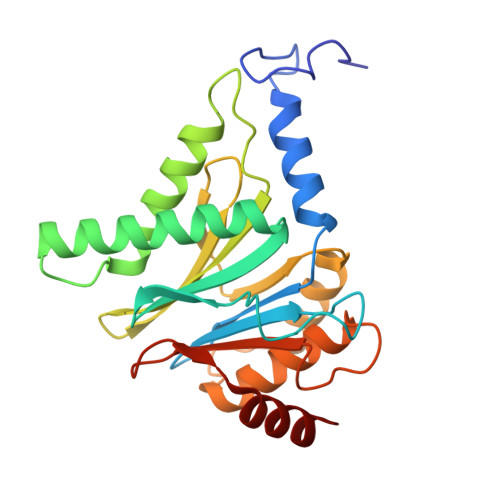
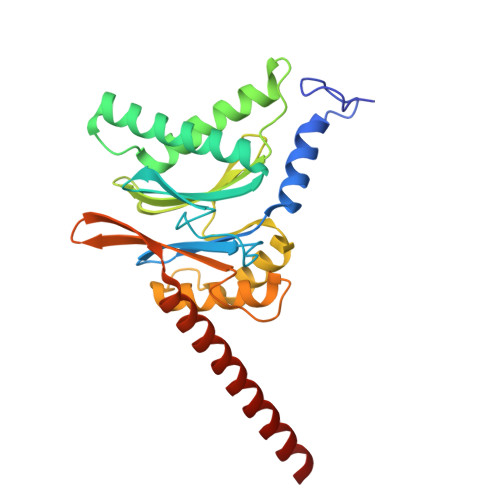
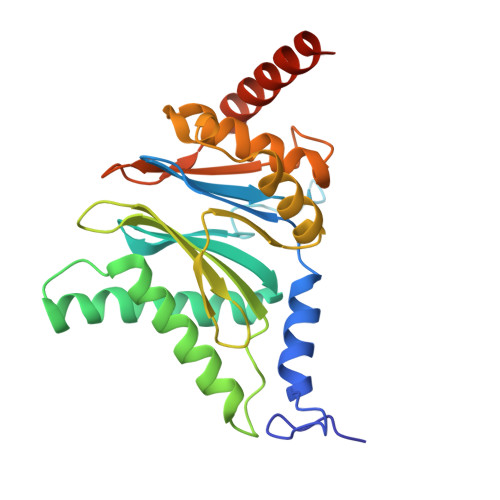
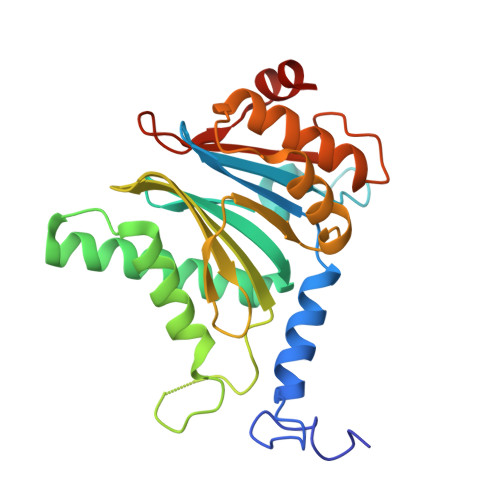
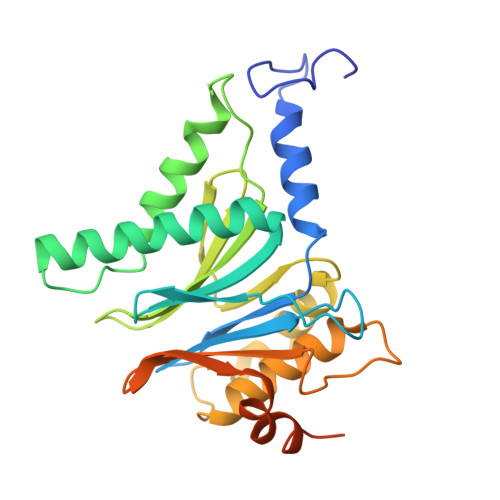
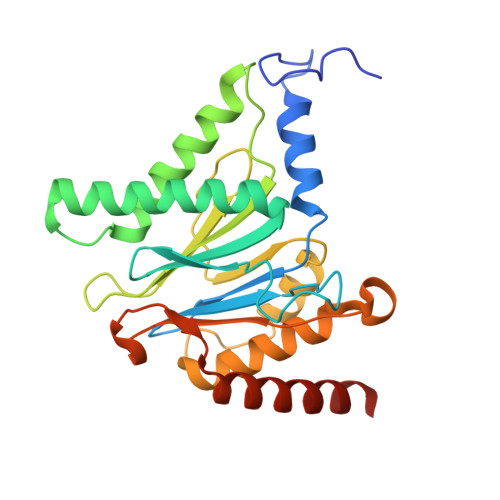
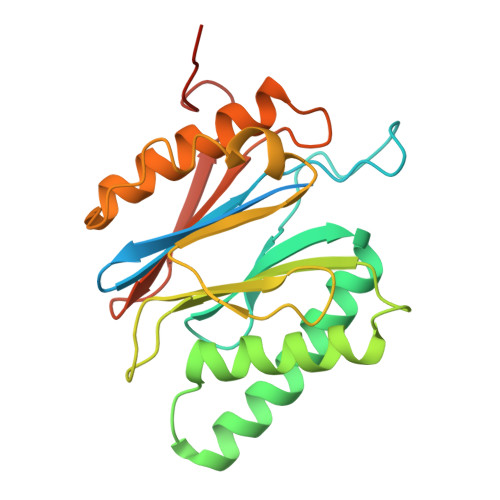
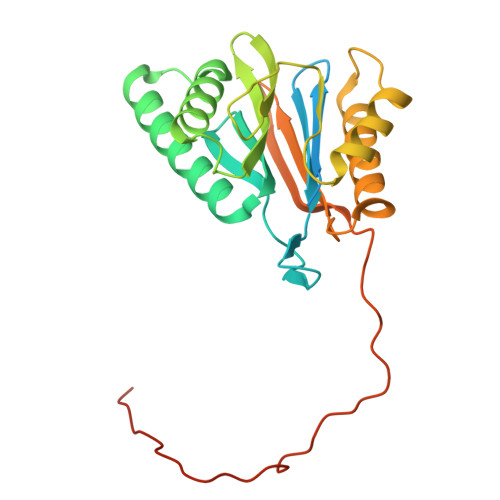
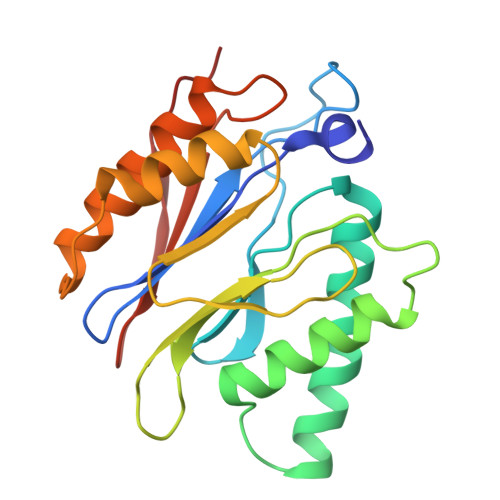
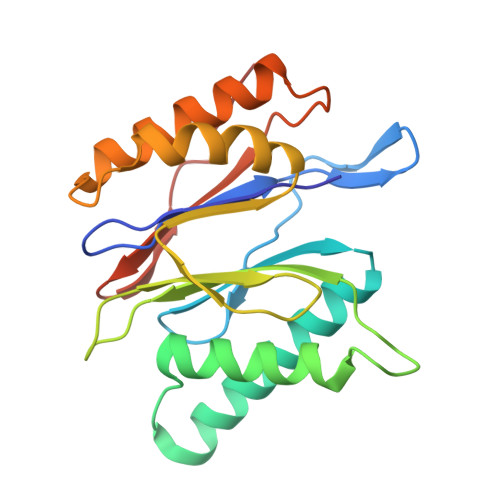
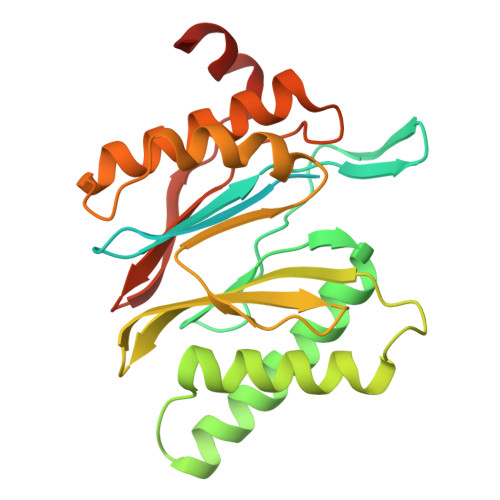
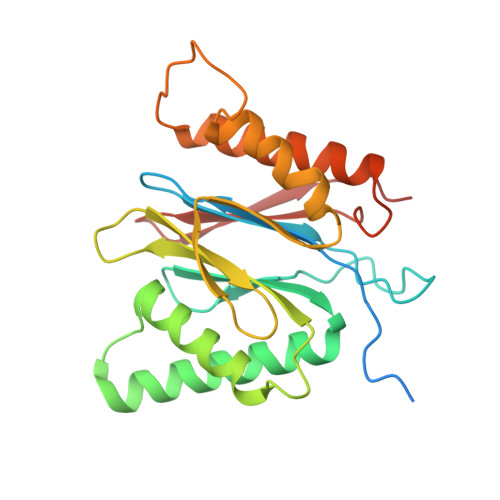
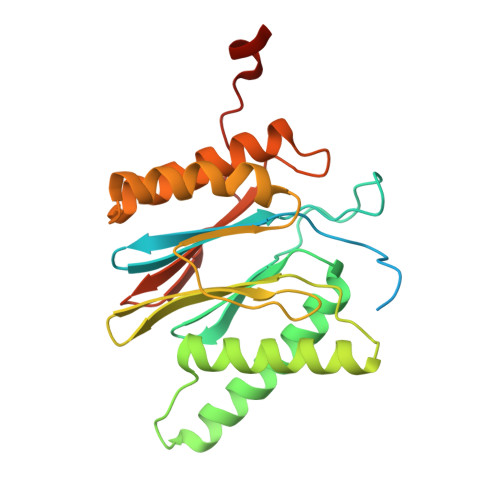
Structure of the TXNL1-bound proteasome.
Gao, J., Nardone, C., Yip, M.C.J., Chino, H., Gu, X., Mirman, Z., Rale, M.J., Paulo, J.A., Elledge, S.J., Shao, S.(2025) Nat Struct Mol Biol 32: 2398-2402
- PubMed: 40770113
- DOI: https://doi.org/10.1038/s41594-025-01639-w
- Primary Citation of Related Structures:
9BW4 - PubMed Abstract:
Proteasomes degrade diverse proteins in different cellular contexts through incompletely defined regulatory mechanisms. Here we report the cryo-EM structure of human thioredoxin-like protein 1 (TXNL1) bound to the 19S regulatory particle of proteasomes via interactions with PSMD1 (Rpn2), PSMD4 (Rpn10) and PSMD14 (Rpn11). Proteasome binding is necessary for the ubiquitin-independent degradation of TXNL1 upon cellular exposure to metal- or metalloid-containing oxidative agents, thereby establishing a structural requirement for the stress-induced degradation of TXNL1.
- Department of Cell Biology, Harvard Medical School, Boston, MA, USA.
Organizational Affiliation: