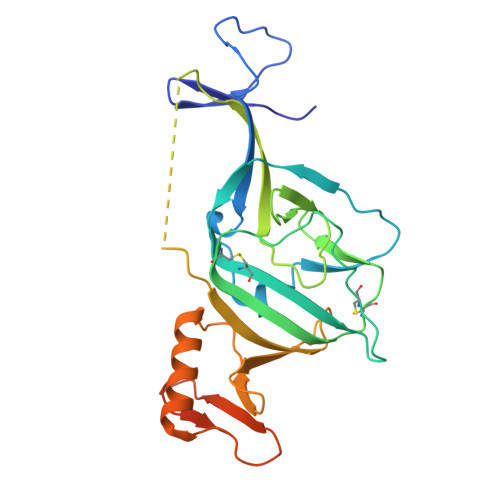
A broadly-neutralizing antibody against Ebolavirus glycoprotein that can potentiate the breadth and neutralization potency of other anti-glycoprotein antibodies
Donnellan, F.R., Rayaprolu, V., Rijal, P., O'Dowd, V., Parvate, A., Callaway, H., Hariharan, C., Parekh, D., Hui, S., Shaffer, K., Hastie, K., Shimanski, L., Muller-Krauter, H., Stecker, T., Balaram, A., Halfmann, P., Saphire, E.O., Lightwood, D.J., Townsend, A.R., Draper, S.J.To be published.