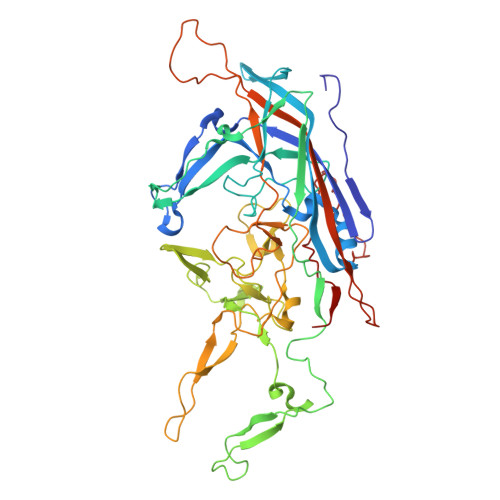
Identification of a novel neutralization epitope in rhesus AAVs.
Dagotto, G., Fisher, J.L., Li, D., Li, Z., Jenni, S., Li, Z., Tartaglia, L.J., Abbink, P., Barouch, D.H.(2024) Mol Ther Methods Clin Dev 32: 101350-101350
- PubMed: 39469420
- DOI: https://doi.org/10.1016/j.omtm.2024.101350
- Primary Citation of Related Structures:
9B52, 9B53 - PubMed Abstract:
Adeno-associated viruses (AAVs) are popular gene therapy delivery vectors, but their application can be limited by anti-vector immunity. Both preexisting neutralizing antibodies (NAbs) and post-administration NAbs can limit transgene expression and reduce the clinical utility of AAVs. The development of novel AAVs will advance our understanding of AAV immunity and may also have practical applications. In this study, we identified five novel AAV capsids from rhesus macaques. RhAAV4282 exhibited 91.4% capsid sequence similarity with AAV7 and showed similar tissue tropism with slightly diminished overall signal. Despite this sequence homology, RhAAV4282 and AAV7 showed limited cross-neutralization. We determined a cryo-EM structure of the RhAAV4282 capsid at 2.57 Å resolution and identified a small segment within the hypervariable region IV, involving seven amino acids that formed a shortened external loop in RhAAV4282 compared with AAV7. We generated RhAAV4282 and AAV7 mutants that involved swaps of this region and showed that this region partially determined neutralization phenotype. We termed this region the hypervariable region IV neutralizing epitope (HRNE). Our data suggests that modification of the HRNE can lead to AAVs with altered neutralization profiles.
- Center for Virology and Vaccine Research, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA 02215, USA.
Organizational Affiliation: