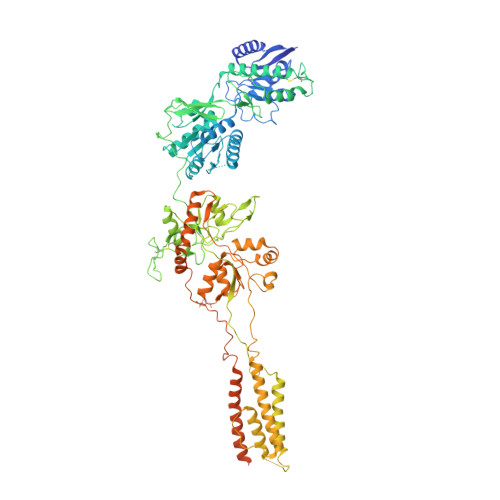
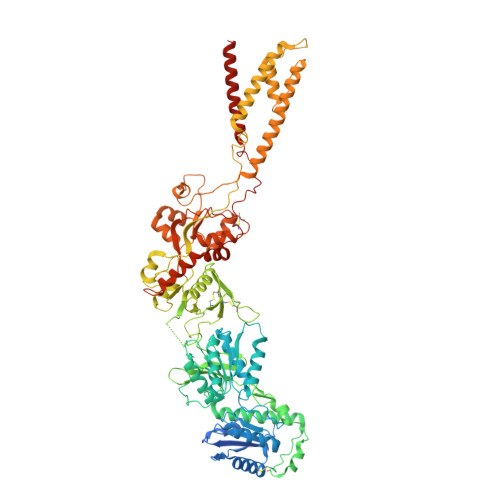
Molecular mechanism of ligand gating and opening of NMDA receptor.
Chou, T.H., Epstein, M., Fritzemeier, R.G., Akins, N.S., Paladugu, S., Ullman, E.Z., Liotta, D.C., Traynelis, S.F., Furukawa, H.(2024) Nature 632: 209-217
- PubMed: 39085540
- DOI: https://doi.org/10.1038/s41586-024-07742-0
- Primary Citation of Related Structures:
9ARE, 9ARF, 9ARG, 9ARH, 9ARI, 9BIB - PubMed Abstract:
Glutamate transmission and activation of ionotropic glutamate receptors are the fundamental means by which neurons control their excitability and neuroplasticity 1 . The N-methyl-D-aspartate receptor (NMDAR) is unique among all ligand-gated channels, requiring two ligands-glutamate and glycine-for activation. These receptors function as heterotetrameric ion channels, with the channel opening dependent on the simultaneous binding of glycine and glutamate to the extracellular ligand-binding domains (LBDs) of the GluN1 and GluN2 subunits, respectively 2,3 . The exact molecular mechanism for channel gating by the two ligands has been unclear, particularly without structures representing the open channel and apo states. Here we show that the channel gate opening requires tension in the linker connecting the LBD and transmembrane domain (TMD) and rotation of the extracellular domain relative to the TMD. Using electron cryomicroscopy, we captured the structure of the GluN1-GluN2B (GluN1-2B) NMDAR in its open state bound to a positive allosteric modulator. This process rotates and bends the pore-forming helices in GluN1 and GluN2B, altering the symmetry of the TMD channel from pseudofourfold to twofold. Structures of GluN1-2B NMDAR in apo and single-liganded states showed that binding of either glycine or glutamate alone leads to distinct GluN1-2B dimer arrangements but insufficient tension in the LBD-TMD linker for channel opening. This mechanistic framework identifies a key determinant for channel gating and a potential pharmacological strategy for modulating NMDAR activity.
- W.M. Keck Structural Biology Laboratory, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, USA.
Organizational Affiliation: