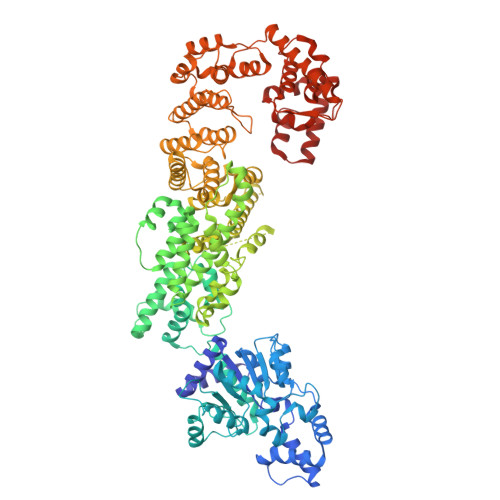
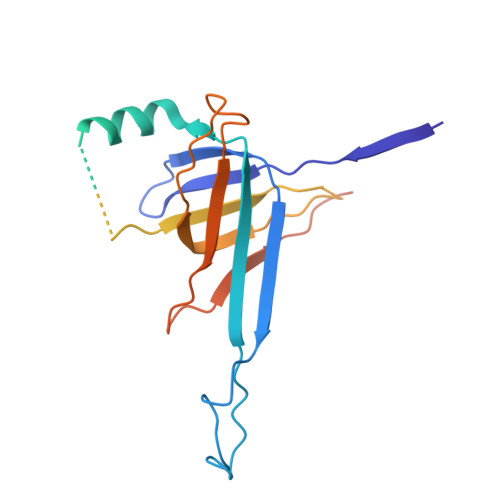
The structural basis of the activation and inhibition of DSR2 NADase by phage proteins.
Wang, R., Xu, Q., Wu, Z., Li, J., Guo, H., Liao, T., Shi, Y., Yuan, L., Gao, H., Yang, R., Shi, Z., Li, F.(2024) Nat Commun 15: 6185-6185
- PubMed: 39039073
- DOI: https://doi.org/10.1038/s41467-024-50410-0
- Primary Citation of Related Structures:
8Y13, 8Y34, 8Y3M, 8Y3W, 8Y3Y, 8ZC9 - PubMed Abstract:
DSR2, a Sir2 domain-containing protein, protects bacteria from phage infection by hydrolyzing NAD + . The enzymatic activity of DSR2 is triggered by the SPR phage tail tube protein (TTP), while suppressed by the SPbeta phage-encoded DSAD1 protein, enabling phages to evade the host defense. However, the molecular mechanisms of activation and inhibition of DSR2 remain elusive. Here, we report the cryo-EM structures of apo DSR2, DSR2-TTP-NAD + and DSR2-DSAD1 complexes. DSR2 assembles into a head-to-head tetramer mediated by its Sir2 domain. The C-terminal helical regions of DSR2 constitute four partner-binding cavities with opened and closed conformation. Two TTP molecules bind to two of the four C-terminal cavities, inducing conformational change of Sir2 domain to activate DSR2. Furthermore, DSAD1 competes with the activator for binding to the C-terminal cavity of DSR2, effectively suppressing its enzymatic activity. Our results provide the mechanistic insights into the DSR2-mediated anti-phage defense system and DSAD1-dependent phage immune evasion.
- MOE Key Laboratory of Rare Pediatric Diseases, Center for Medical Genetics, School of Life Sciences, Central South University, Changsha, Hunan, China.
Organizational Affiliation: