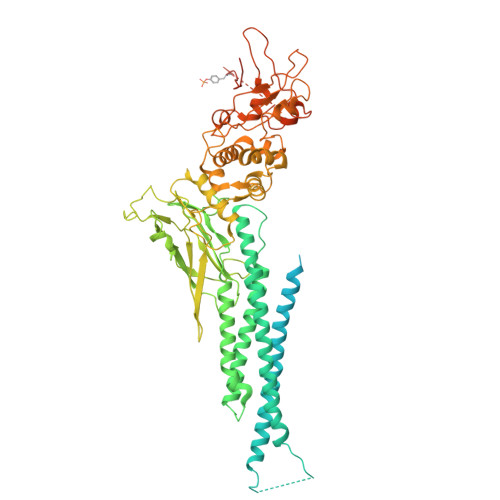
Structural analysis reveals how tetrameric tyrosine-phosphorylated STAT1 is targeted by the rabies virus P-protein.
Sugiyama, A., Minami, M., Ugajin, K., Inaba-Inoue, S., Yabuno, N., Takekawa, Y., Xiaomei, S., Takei, S., Sasaki, M., Nomai, T., Jiang, X., Kita, S., Maenaka, K., Hirose, M., Yao, M., Gooley, P.R., Moseley, G.W., Sugita, Y., Ose, T.(2025) Sci Signal 18: eads2210-eads2210
- PubMed: 40100957
- DOI: https://doi.org/10.1126/scisignal.ads2210
- Primary Citation of Related Structures:
8YYU, 8YYV - PubMed Abstract:
Signal transducer and activator of transcription (STAT) family members mediate signaling in the Janus kinase (JAK)-STAT pathway and are activated by phosphorylation at a conserved tyrosine residue, resulting in dimerization through reciprocal interactions between the phosphotyrosine and a Src homology 2 (SH2) domain. Tyrosine-phosphorylated STAT (pY-STAT) then translocates to the nucleus to induce the expression of genes encoding antiviral proteins. Although the active and functional forms of STATs are conventionally considered to be dimers, STATs can undergo higher-order oligomerization, which is implicated in regulating transcriptional activity. We present the cryo-electron microscopy (cryo-EM) structure of the tetrameric form of intact pY-STAT1 in complex with DNA, which indicates that interactions between the amino-terminal domains (NTDs) of STAT1 induce oligomerization. The tetrameric structure revealed a compact conformation with a previously uncharacterized binding interface: Two DNA-bound dimers are twofold symmetrically aligned to transform into a tandem DNA-binding model without NTD dimer separation. Moreover, biochemical analyses indicated that the rabies virus P-protein selectively targeted tetrameric pY-STAT1. Combined with data showing which regions contribute to the interaction between pY-STAT1 and the P-protein, we constructed a binding model explaining how P recognizes the pY-STAT1 tetramer. These data provide insight into how pathogenic viruses target signaling pathways that mediate the host immune response.
- Faculty of Advanced Life Science, Hokkaido University, Sapporo 060-0810, Japan.
Organizational Affiliation: