Structure of human LGI1-ADAM22 complex in space group P212121
Liu, H., Xu, F.To be published.
Experimental Data Snapshot
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Disintegrin and metalloproteinase domain-containing protein 22 | A, C [auth B], D [auth C] | 486 | Homo sapiens | Mutation(s): 0 Gene Names: ADAM22, MDC2 | 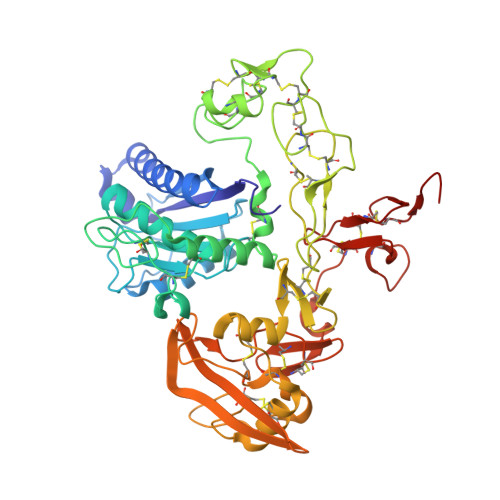 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q9P0K1 (Homo sapiens) Explore Q9P0K1 Go to UniProtKB: Q9P0K1 | |||||
PHAROS: Q9P0K1 GTEx: ENSG00000008277 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9P0K1 | ||||
Glycosylation | |||||
| Glycosylation Sites: 3 | Go to GlyGen: Q9P0K1-1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Leucine-rich glioma-inactivated protein 1 | B [auth D], E [auth F], F [auth E] | 526 | Homo sapiens | Mutation(s): 0 Gene Names: LGI1, EPT, UNQ775/PRO1569 | 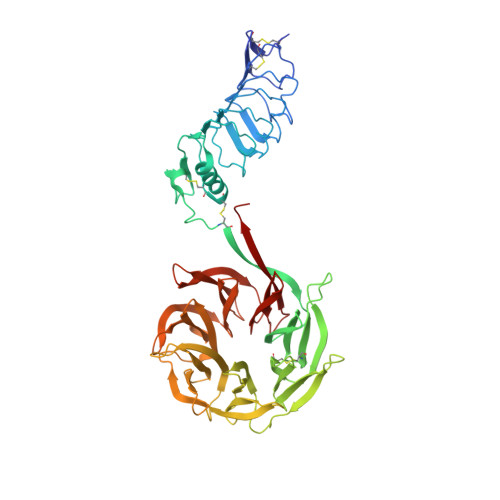 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for O95970 (Homo sapiens) Explore O95970 Go to UniProtKB: O95970 | |||||
PHAROS: O95970 GTEx: ENSG00000108231 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O95970 | ||||
Glycosylation | |||||
| Glycosylation Sites: 3 | Go to GlyGen: O95970-1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| NAG Query on NAG | CA [auth F] DA [auth F] EA [auth F] G [auth A] GA [auth E] | 2-acetamido-2-deoxy-beta-D-glucopyranose C8 H15 N O6 OVRNDRQMDRJTHS-FMDGEEDCSA-N |  | ||
| CA Query on CA | AA [auth C] BA [auth C] FA [auth F] J [auth A] JA [auth E] | CALCIUM ION Ca BHPQYMZQTOCNFJ-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 55.448 | α = 90 |
| b = 196.078 | β = 90 |
| c = 420.75 | γ = 90 |
| Software Name | Purpose |
|---|---|
| HKL-2000 | data scaling |
| PHASER | phasing |
| PHENIX | refinement |
| HKL-2000 | data reduction |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Science Foundation (NSF, China) | China | 31270767 |