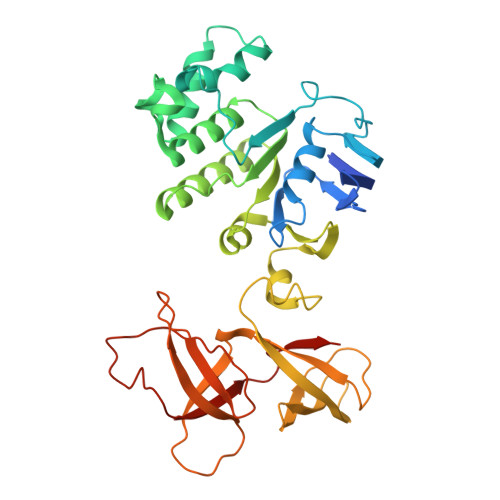
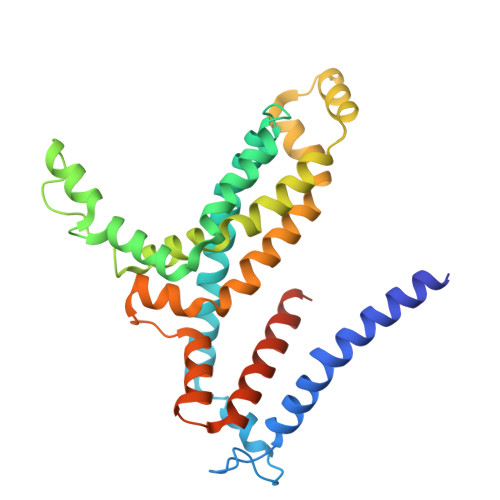
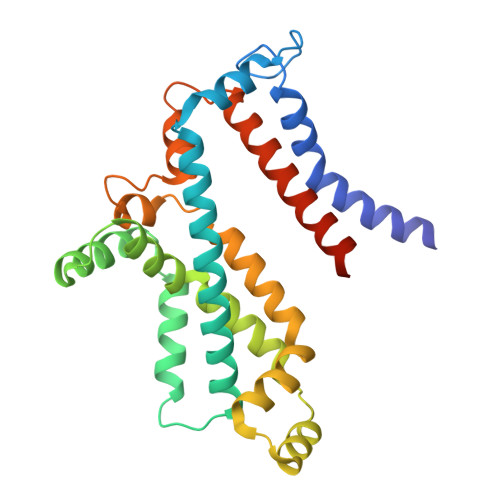
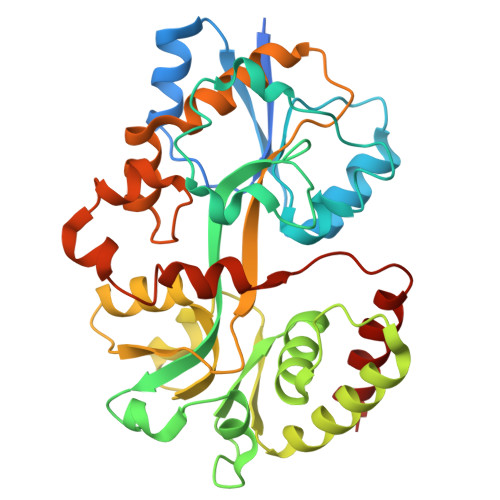
Structural insights into polyamine spermidine uptake by the ABC transporter PotD-PotABC.
Qiao, Z., Do, P.H., Yeo, J.Y., Ero, R., Li, Z., Zhan, L., Basak, S., Gao, Y.G.(2024) Sci Adv 10: eado8107-eado8107
- PubMed: 39303029
- DOI: https://doi.org/10.1126/sciadv.ado8107
- Primary Citation of Related Structures:
8Y5F, 8Y5G, 8Y5H, 8Y5I, 8ZX1 - PubMed Abstract:
Polyamines, characterized by their polycationic nature, are ubiquitously present in all organisms and play numerous cellular functions. Among polyamines, spermidine stands out as the predominant type in both prokaryotic and eukaryotic cells. The PotD-PotABC protein complex in Escherichia coli , belonging to the adenosine triphosphate-binding cassette transporter family, is a spermidine-preferential uptake system. Here, we report structural details of the polyamine uptake system PotD-PotABC in various states. Our analyses reveal distinct "inward-facing" and "outward-facing" conformations of the PotD-PotABC transporter, as well as conformational changes in the "gating" residues (F222, Y223, D226, and K241 in PotB; Y219 and K223 in PotC) controlling spermidine uptake. Therefore, our structural analysis provides insights into how the PotD-PotABC importer recognizes the substrate-binding protein PotD and elucidates molecular insights into the spermidine uptake mechanism of bacteria.
- School of Biological Sciences, Nanyang Technological University, Singapore 637551, Singapore.
Organizational Affiliation: