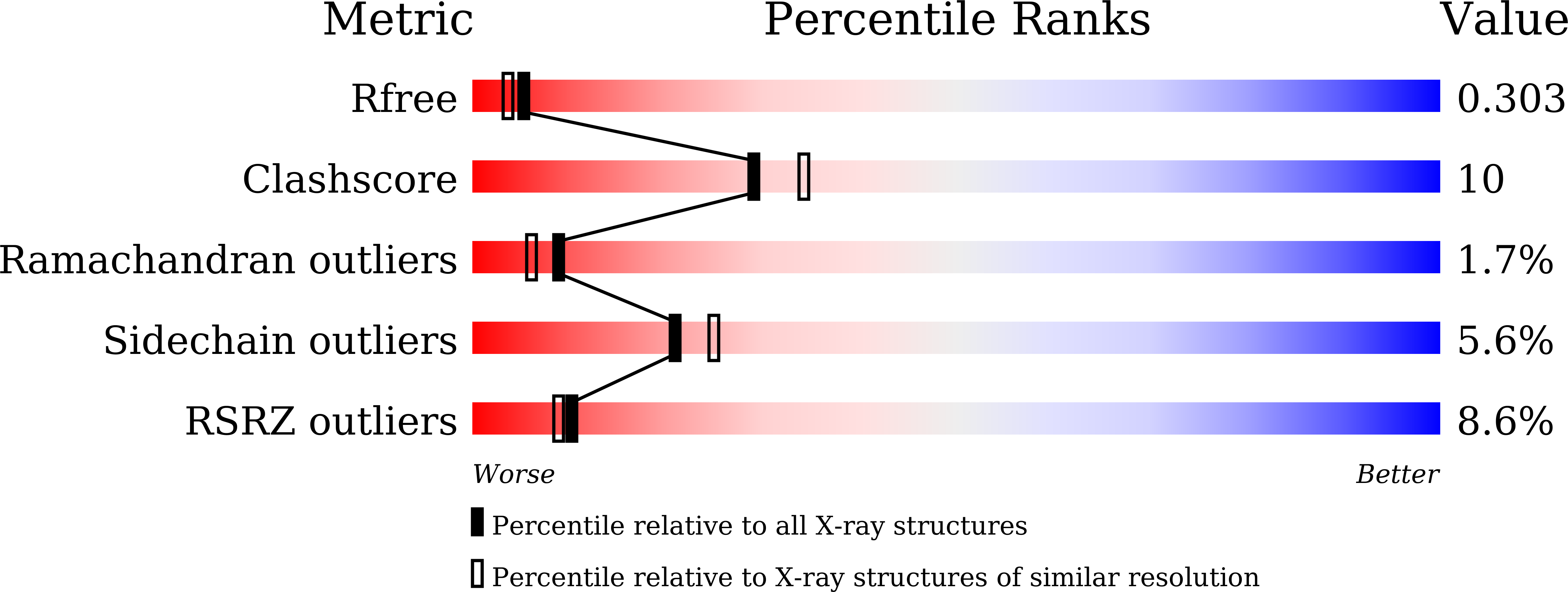
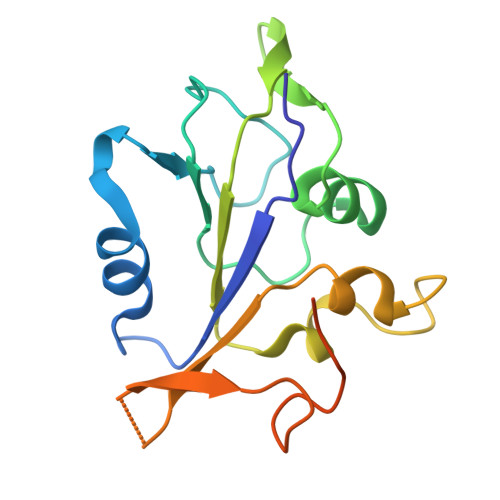
Crystal structure of the ATPase domain of porcine circovirus type 2 Rep protein.
Guan, S., Li, Z., Han, Y., Tian, A., Zhou, S., Chen, H., Peng, G., Song, Y.(2024) J Gen Virol 105
- PubMed: 38506716
- DOI: https://doi.org/10.1099/jgv.0.001972
- Primary Citation of Related Structures:
8XMS - PubMed Abstract:
PCV2 belongs to the genus Circovirus in the family Circoviridae , whose genome is replicated by rolling circle replication (RCR). PCV2 Rep is a multifunctional enzyme that performs essential functions at multiple stages of viral replication. Rep is responsible for nicking and ligating single-stranded DNA and unwinding double-stranded DNA (dsDNA). However, the structure and function of the Rep are still poorly understood, which significantly impedes viral replication research. This study successfully resolved the structure of the PCV2 Rep ATPase domain (PRAD) using X-ray crystallography. Homologous structure search revealed that Rep belonged to the superfamily 3 (SF3) helicase, and multiple conserved residues were identified during sequence alignment with SF3 family members. Simultaneously, a hexameric PRAD model was generated for analysing characteristic structures and sites. Mutation of the conserved site and measurement of its activity showed that the hallmark motifs of the SF3 family influenced helicase activity by affecting ATPase activity and β-hairpin just caused the loss of helicase activity. The structural and functional analyses of the PRAD provide valuable insights for future research on PCV2 replication and antiviral strategies.
Organizational Affiliation:
State Key Laboratory of Agricultural Microbiology, Huazhong Agricultural University, Wuhan, 430070, PR China.