Structural plasticity of human leptin binding to its receptor LepR
Xie, Y.F., Li, X., Qi, J., Shang, G., Lu, D., Gao, G.F.(2023) Hlife 1: 115-123
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Leptin receptor | A, B, F [auth C] | 829 | Homo sapiens | Mutation(s): 0 Gene Names: LEPR, DB, OBR | 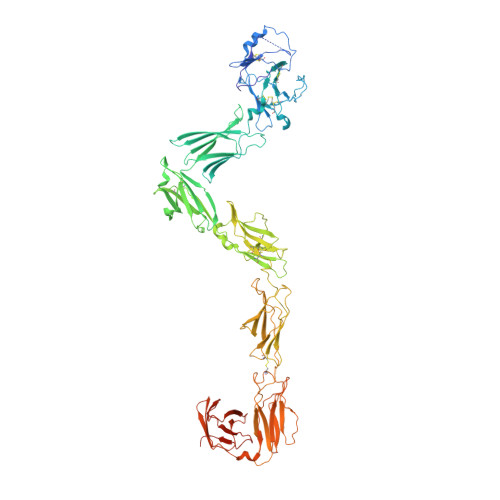 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P48357 (Homo sapiens) Explore P48357 Go to UniProtKB: P48357 | |||||
PHAROS: P48357 GTEx: ENSG00000116678 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P48357 | ||||
Glycosylation | |||||
| Glycosylation Sites: 7 | Go to GlyGen: P48357-1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Leptin | C [auth D], D [auth F], E | 167 | Homo sapiens | Mutation(s): 0 Gene Names: LEP | 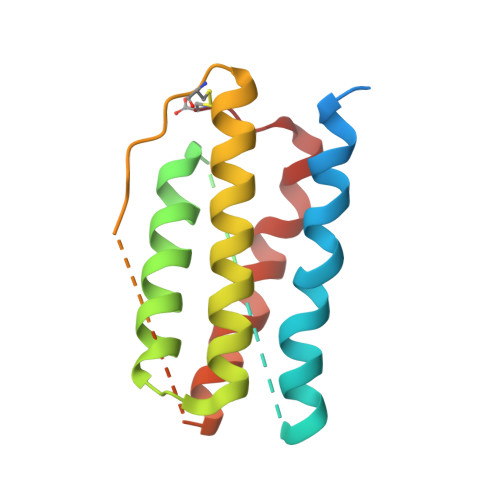 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P41159 (Homo sapiens) Explore P41159 Go to UniProtKB: P41159 | |||||
PHAROS: P41159 GTEx: ENSG00000174697 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P41159 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| NAG Query on NAG | H [auth A] I [auth A] J [auth A] K [auth A] L [auth A] | 2-acetamido-2-deoxy-beta-D-glucopyranose C8 H15 N O6 OVRNDRQMDRJTHS-FMDGEEDCSA-N |  | ||
| Task | Software Package | Version |
|---|---|---|
| MODEL REFINEMENT | PHENIX | |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Natural Science Foundation of China (NSFC) | China | -- |