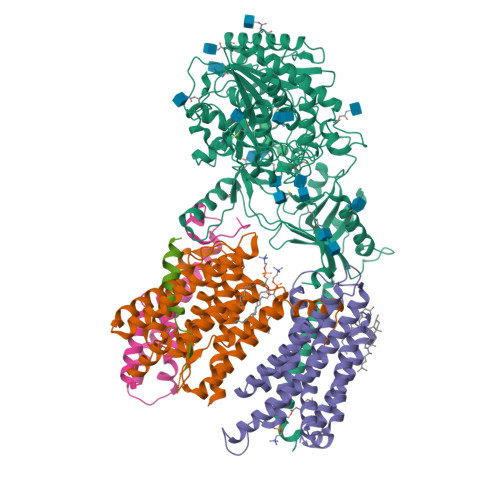
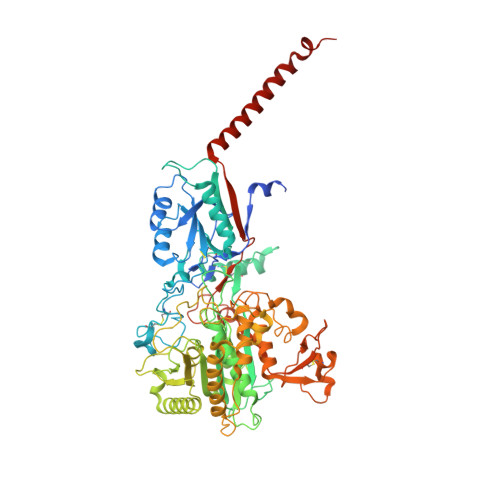
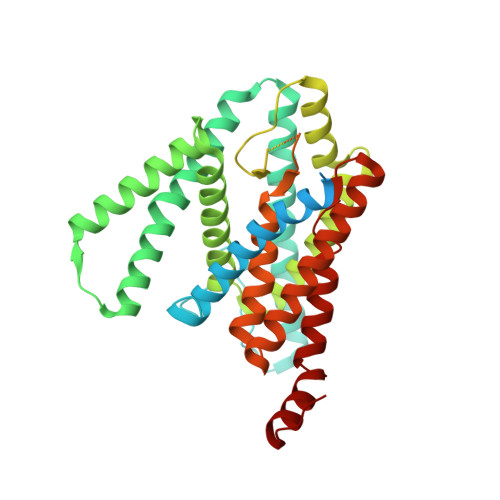
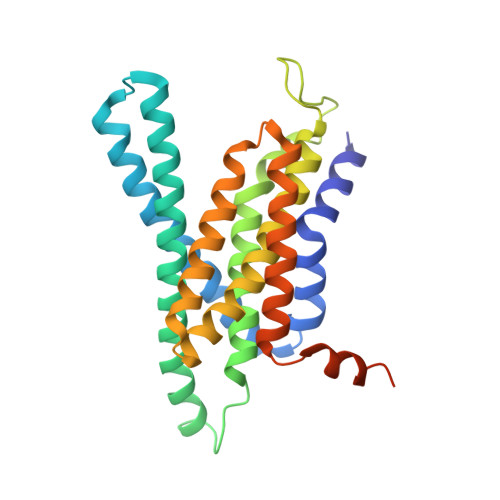
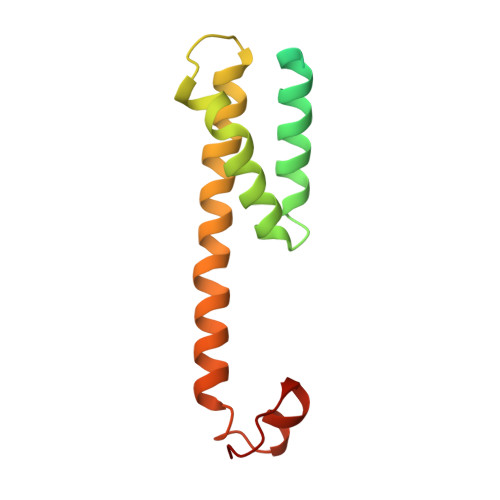
Molecular mechanism of substrate recognition and cleavage by human gamma-secretase.
Guo, X., Li, H., Yan, C., Lei, J., Zhou, R., Shi, Y.(2024) Science 384: 1091-1095
- PubMed: 38843321
- DOI: https://doi.org/10.1126/science.adn5820
- Primary Citation of Related Structures:
8X52, 8X53, 8X54 - PubMed Abstract:
Successive cleavages of amyloid precursor protein C-terminal fragment with 99 residues (APP-C99) by γ-secretase result in amyloid-β (Aβ) peptides of varying lengths. Most cleavages have a step size of three residues. To elucidate the underlying mechanism, we determined the atomic structures of human γ-secretase bound individually to APP-C99, Aβ49, Aβ46, and Aβ43. In all cases, the substrate displays the same structural features: a transmembrane α-helix, a three-residue linker, and a β-strand that forms a hybrid β-sheet with presenilin 1 (PS1). Proteolytic cleavage occurs just ahead of the substrate β-strand. Each cleavage is followed by unwinding and translocation of the substrate α-helix by one turn and the formation of a new β-strand. This mechanism is consistent with existing biochemical data and may explain the cleavages of other substrates by γ-secretase.
Organizational Affiliation:
Beijing Frontier Research Center for Biological Structure, Tsinghua-Peking Joint Center for Life Sciences, Key Laboratory for Protein Sciences of Ministry of Education, School of Life Sciences, Tsinghua University, Beijing 100084, China.