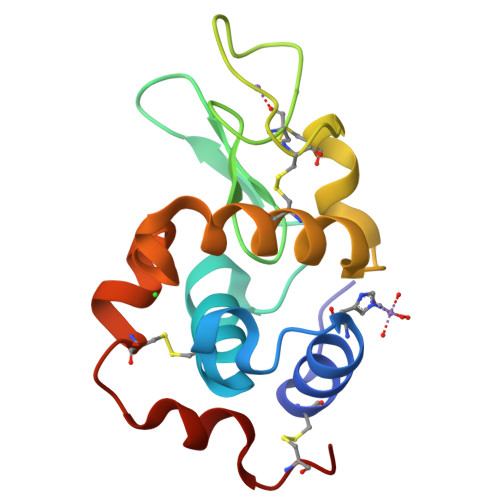
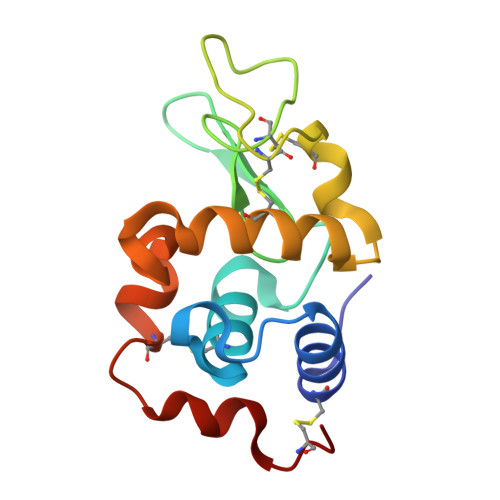
Real-time observation of a metal complex-driven reaction intermediate using a porous protein crystal and serial femtosecond crystallography.
Maity, B., Shoji, M., Luo, F., Nakane, T., Abe, S., Owada, S., Kang, J., Tono, K., Tanaka, R., Pham, T.T., Kojima, M., Hishikawa, Y., Tanaka, J., Tian, J., Nagama, M., Suzuki, T., Noya, H., Nakasuji, Y., Asanuma, A., Yao, X., Iwata, S., Shigeta, Y., Nango, E., Ueno, T.(2024) Nat Commun 15: 5518-5518
- PubMed: 38951539
- DOI: https://doi.org/10.1038/s41467-024-49814-9
- Primary Citation of Related Structures:
8WZF, 8WZG, 8WZR, 8WZT, 8WZV - PubMed Abstract:
Determining short-lived intermediate structures in chemical reactions is challenging. Although ultrafast spectroscopic methods can detect the formation of transient intermediates, real-space structures cannot be determined directly from such studies. Time-resolved serial femtosecond crystallography (TR-SFX) has recently proven to be a powerful method for capturing molecular changes in proteins on femtosecond timescales. However, the methodology has been mostly applied to natural proteins/enzymes and limited to reactions promoted by synthetic molecules due to structure determination challenges. This work demonstrates the applicability of TR-SFX for investigations of chemical reaction mechanisms of synthetic metal complexes. We fix a light-induced CO-releasing Mn(CO) 3 reaction center in porous hen egg white lysozyme (HEWL) microcrystals. By controlling light exposure and time, we capture the real-time formation of Mn-carbonyl intermediates during the CO release reaction. The asymmetric protein environment is found to influence the order of CO release. The experimentally-observed reaction path agrees with quantum mechanical calculations. Therefore, our demonstration offers a new approach to visualize atomic-level reactions of small molecules using TR-SFX with real-space structure determination. This advance holds the potential to facilitate design of artificial metalloenzymes with precise mechanisms, empowering design, control and development of innovative reactions.
Organizational Affiliation:
School of Life Science and Technology, Tokyo Institute of Technology, Nagatsuta-cho 4259, Midori-ku, Yokohama, Japan. basudev@bio.titech.ac.jp.