Aldo-keto reductase KmAKR - T23V/Y28A/K29H/T63M/Q213A/Y296W/W297H from Kluyveromyces marxianus
Xu, S.Y., Zhou, L., Xu, Y., Wang, Y.J., Zheng, Y.G.To be published.
Experimental Data Snapshot
Starting Model: in silico
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| NADPH-dependent alpha-keto amide reductase | 310 | Kluyveromyces marxianus | Mutation(s): 7 Gene Names: KLMA_60481 | 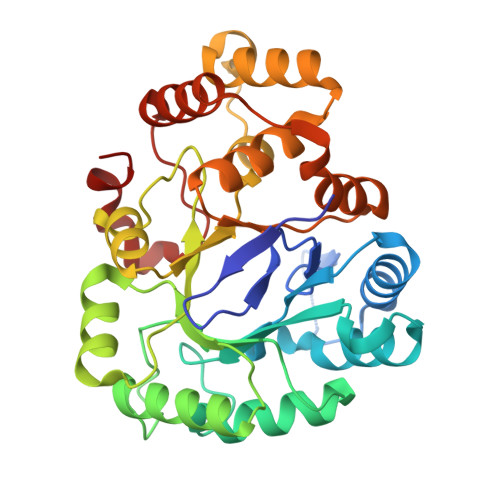 | |
UniProt | |||||
Find proteins for W0TDP7 (Kluyveromyces marxianus (strain DMKU3-1042 / BCC 29191 / NBRC 104275)) Explore W0TDP7 Go to UniProtKB: W0TDP7 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | W0TDP7 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 67.666 | α = 90 |
| b = 115.182 | β = 101.46 |
| c = 83.461 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| Aimless | data scaling |
| DIALS | data reduction |
| MOLREP | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Natural Science Foundation of China (NSFC) | China | 22178318 |