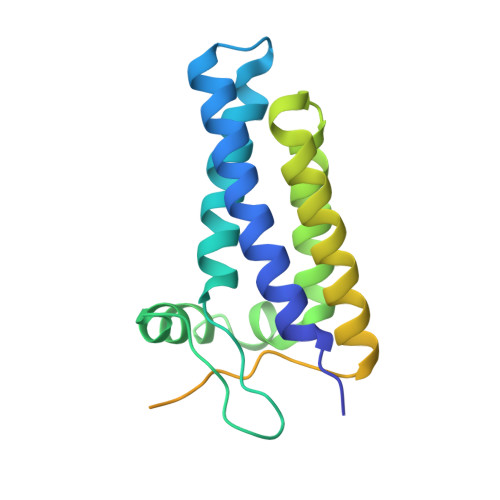
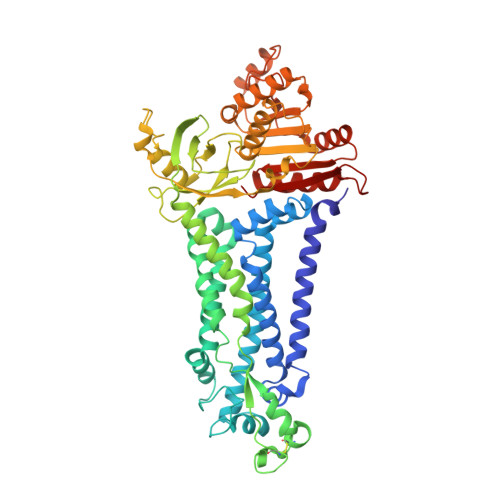
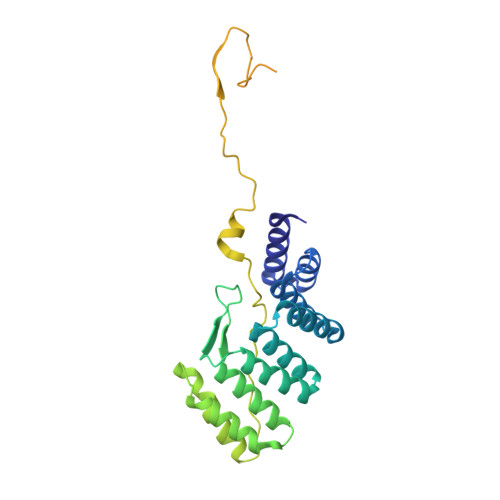
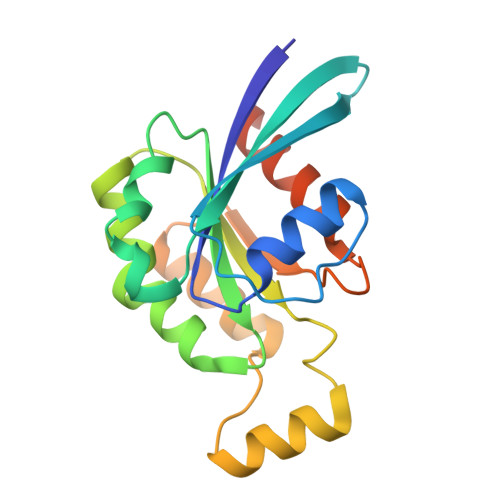
Structure of human phagocyte NADPH oxidase in the activated state.
Liu, X., Shi, Y., Liu, R., Song, K., Chen, L.(2024) Nature 627: 189-195
- PubMed: 38355798
- DOI: https://doi.org/10.1038/s41586-024-07056-1
- Primary Citation of Related Structures:
8WEJ, 8X2L - PubMed Abstract:
Phagocyte NADPH oxidase, a protein complex with a core made up of NOX2 and p22 subunits, is responsible for transferring electrons from intracellular NADPH to extracellular oxygen 1 . This process generates superoxide anions that are vital for killing pathogens 1 . The activation of phagocyte NADPH oxidase requires membrane translocation and the binding of several cytosolic factors 2 . However, the exact mechanism by which cytosolic factors bind to and activate NOX2 is not well understood. Here we present the structure of the human NOX2-p22 complex activated by fragments of three cytosolic factors: p47, p67 and Rac1. The structure reveals that the p67-Rac1 complex clamps onto the dehydrogenase domain of NOX2 and induces its contraction, which stabilizes the binding of NADPH and results in a reduction of the distance between the NADPH-binding domain and the flavin adenine dinucleotide (FAD)-binding domain. Furthermore, the dehydrogenase domain docks onto the bottom of the transmembrane domain of NOX2, which reduces the distance between FAD and the inner haem. These structural rearrangements might facilitate the efficient transfer of electrons between the redox centres in NOX2 and lead to the activation of phagocyte NADPH oxidase.
- State Key Laboratory of Membrane Biology, College of Future Technology, Institute of Molecular Medicine, Peking University, Beijing Key Laboratory of Cardiometabolic Molecular Medicine, Beijing, China.
Organizational Affiliation: