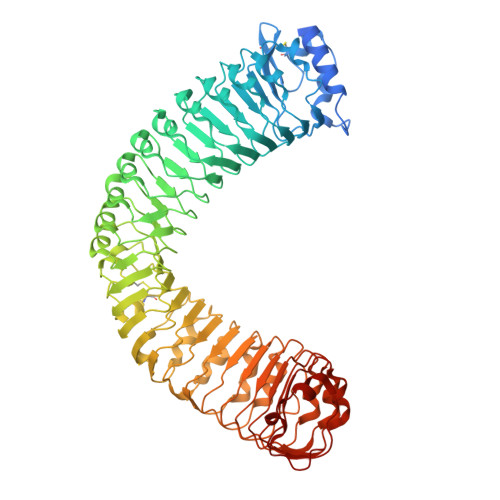
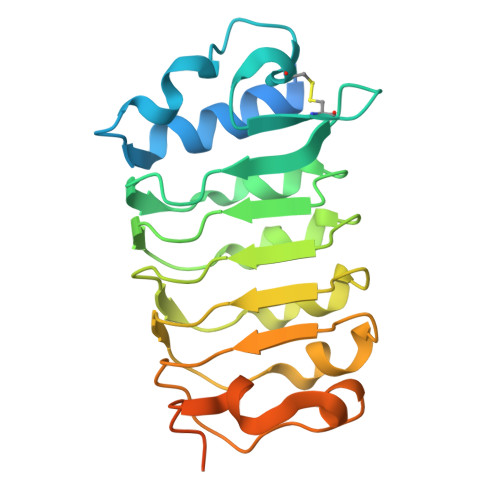
Mechanistic study of SCOOPs recognition by MIK2-BAK1 complex reveals the role of N-glycans in plant ligand-receptor-coreceptor complex formation.
Wu, H., Wan, L., Liu, Z., Jian, Y., Zhang, C., Mao, X., Wang, Z., Wang, Q., Hu, Y., Xiong, L., Xia, Z., Xue, J., Li, S., He, P., Shan, L., Xu, S.(2024) Nat Plants 10: 1984-1998
- PubMed: 39511418
- DOI: https://doi.org/10.1038/s41477-024-01836-3
- Primary Citation of Related Structures:
8WEB, 8WEC, 8WED, 8WEE, 8WEF, 8WEG, 8WEH, 8WEI - PubMed Abstract:
Ligand-induced receptor and co-receptor heterodimerization is a common mechanism in receptor kinase (RK) signalling activation. SERINE-RICH ENDOGENOUS PEPTIDEs (SCOOPs) mediate the complex formation of Arabidopsis RK MIK2 and co-receptor BAK1, triggering immune responses. Through structural, biochemical and genetic analyses, we demonstrate that SCOOPs use their SxS motif and adjacent residues to bind MIK2 and the carboxy-terminal GGR residues to link MIK2 to BAK1. While N-glycosylation of plant RKs is typically associated with protein maturation, plasma membrane targeting and conformation maintenance, a surprising revelation emerges from our crystal structural analysis of MIK2-SCOOP-BAK1 complexes. Specific N-glycans on MIK2 directly interact with BAK1 upon SCOOP sensing. The absence of N-glycosylation at the specific site in MIK2 neither affects its subcellular localization and protein accumulation in plant cells nor alters its structural conformation, but markedly reduces its affinity for BAK1, abolishing SCOOP-triggered immune responses. This N-glycan-mediated receptor and co-receptor heterodimerization occurs in both Arabidopsis and Brassica napus. Our findings elucidate the molecular basis of SCOOP perception by the MIK2-BAK1 immune complex and underscore the crucial role of N-glycans in plant receptor-coreceptor interactions and signalling activation, shaping immune responses.
- National Key Lab of Agricultural Microbiology, Hubei Hongshan Laboratory, College of Life Science and Technology, Huazhong Agricultural University, Wuhan, China.
Organizational Affiliation: