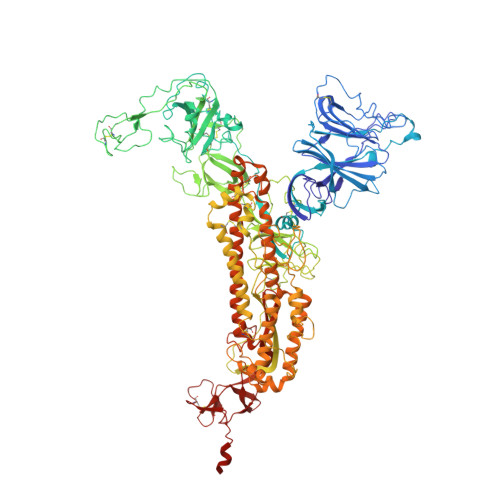
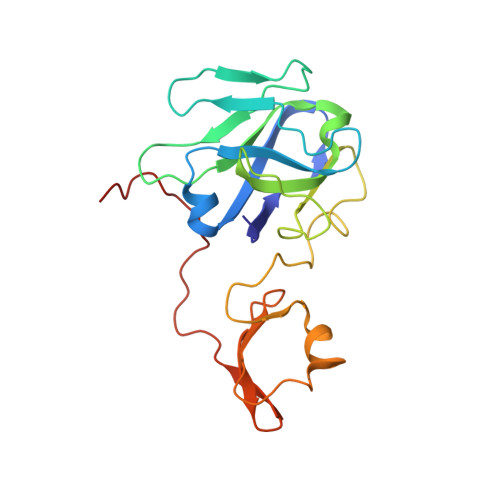
Structure-guided design of a trivalent nanobody cluster targeting SARS-CoV-2 spike protein.
Jiang, X., Qin, Q., Zhu, H., Qian, J., Huang, Q.(2024) Int J Biol Macromol 256: 128191-128191
- PubMed: 38000614
- DOI: https://doi.org/10.1016/j.ijbiomac.2023.128191
- Primary Citation of Related Structures:
8W4F - PubMed Abstract:
Nanobodies are natural anti-SARS-CoV-2 drug candidates. Engineering multivalent nanobodies is an effective way to improve the functional binding affinity of natural nanobodies by simultaneously targeting multiple sites on viral proteins. However, multivalent nanobodies have usually been engineered by trial and error, and rational designs are still lacking. Here, we describe a structure-guided design of a self-assembled trivalent nanobody cluster targeting the SARS-CoV-2 spike protein. Using the nanobody Nb6 as a monovalent binder, we first selected a human-derived trimerization scaffold evaluated by molecular dynamics simulations, then selected an optimal linker according to the minimum distance between Nb6 and the trimerization scaffold, and finally successfully engineered a trivalent nanobody cluster called Tribody. Compared with the low-affinity monovalent counterpart (Nb6), Tribody showed much higher target binding affinity (K D < 1 pM) and thus had a 900-fold increase in antiviral neutralization against SARS-CoV-2 pseudovirus. We determined the cryo-EM structure of the Tribody-spike complex and confirmed that all three Nb6 binders of Tribody collectively bind to the three receptor-binding domains (RBDs) of the spike and lock them in a 3-RBD-down conformation, fully consistent with our structure-guided design. This study demonstrates that synthetic nanobody clusters with human-derived self-assembled scaffolds are potential protein drugs against SARS-CoV-2 coronaviruses.
- State Key Laboratory of Genetic Engineering, Shanghai Engineering Research Center of Industrial Microorganisms, MOE Engineering Research Center of Gene Technology, School of Life Sciences, Fudan University, Shanghai 200438, China.
Organizational Affiliation: