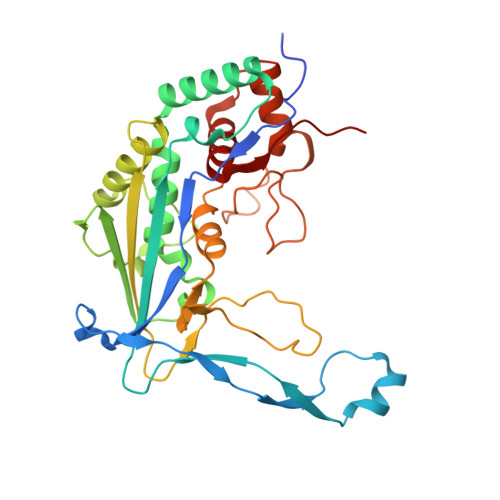
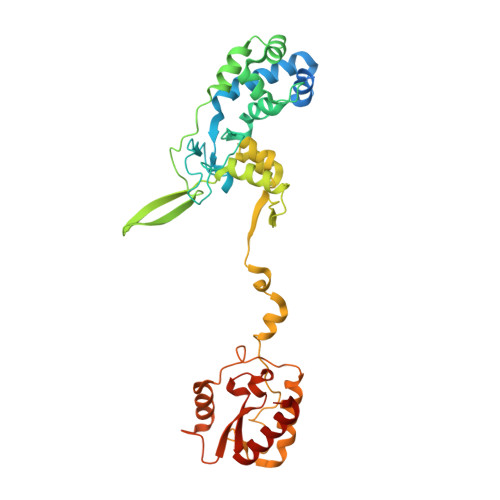
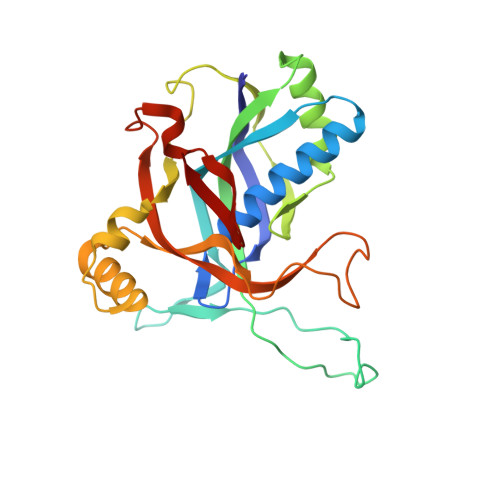
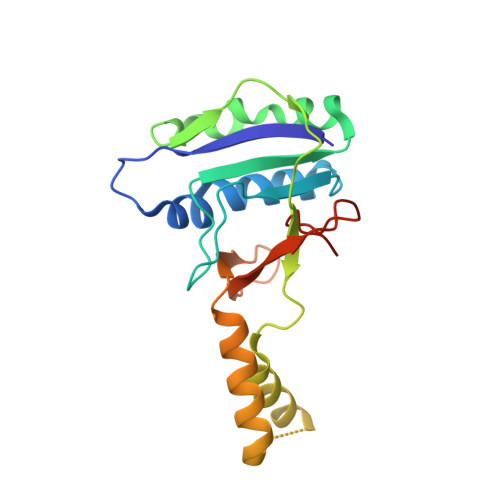
Structural determinants of DNA cleavage by a CRISPR HNH-Cascade system.
Hirano, S., Altae-Tran, H., Kannan, S., Macrae, R.K., Zhang, F.(2024) Mol Cell 84: 3154
- PubMed: 39111310
- DOI: https://doi.org/10.1016/j.molcel.2024.07.026
- Primary Citation of Related Structures:
8W1P - PubMed Abstract:
Canonical prokaryotic type I CRISPR-Cas adaptive immune systems contain a multicomponent effector complex called Cascade, which degrades large stretches of DNA via Cas3 helicase-nuclease activity. Recently, a highly precise subtype I-F1 CRISPR-Cas system (HNH-Cascade) was found that lacks Cas3, the absence of which is compensated for by the insertion of an HNH endonuclease domain in the Cas8 Cascade component. Here, we describe the cryo-EM structure of Selenomonas sp. HNH-Cascade (SsCascade) in complex with target DNA and characterize its mechanism of action. The Cascade scaffold is complemented by the HNH domain, creating a ring-like structure in which the unwound target DNA is precisely cleaved. This structure visualizes a unique hybrid of two extensible biological systems-Cascade, an evolutionary platform for programmable DNA effectors, and an HNH nuclease, an adaptive domain with a spectrum of enzymatic activity.
- Broad Institute of MIT and Harvard, Cambridge, MA 02142, USA; McGovern Institute for Brain Research at MIT, Cambridge, MA 02139, USA; Department of Brain and Cognitive Science, Massachusetts Institute of Technology, Cambridge, MA 02139, USA; Department of Biological Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139, USA; Howard Hughes Medical Institute, Cambridge, MA 02139, USA.
Organizational Affiliation: