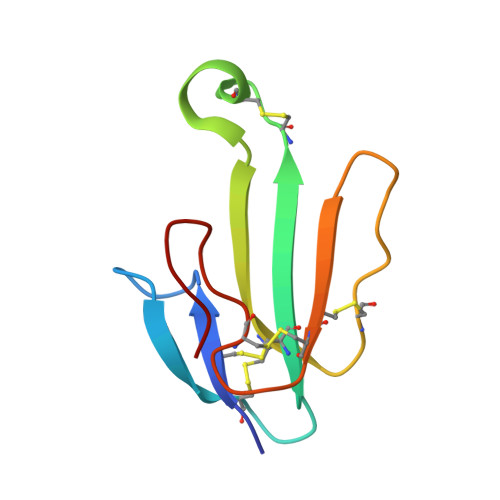
Scalable production of recombinant three-finger proteins: from inclusion bodies to high quality molecular probes.
Xu, J., Lei, X., Li, A., Li, J., Li, S., Chen, L.(2024) Microb Cell Fact 23: 48-48
- PubMed: 38347541
- DOI: https://doi.org/10.1186/s12934-024-02316-1
- Primary Citation of Related Structures:
8VY8 - PubMed Abstract:
The three-finger proteins are a collection of disulfide bond rich proteins of great biomedical interests. Scalable recombinant expression and purification of bioactive three-finger proteins is quite difficult. We introduce a working pipeline for expression, purification and validation of disulfide-bond rich three-finger proteins using E. coli as the expression host. With this pipeline, we have successfully obtained highly purified and bioactive recombinant α-Βungarotoxin, k-Bungarotoxin, Hannalgesin, Mambalgin-1, α-Cobratoxin, MTα, Slurp1, Pate B etc. Milligrams to hundreds of milligrams of recombinant three finger proteins were obtained within weeks in the lab. The recombinant proteins showed specificity in binding assay and six of them were crystallized and structurally validated using X-ray diffraction protein crystallography. Our pipeline allows refolding and purifying recombinant three finger proteins under optimized conditions and can be scaled up for massive production of three finger proteins. As many three finger proteins have attractive therapeutic or research interests and due to the extremely high quality of the recombinant three finger proteins we obtained, our method provides a competitive alternative to either their native counterparts or chemically synthetic ones and should facilitate related research and applications.
- Molecular and Computational Biology, Department of Biological Sciences, University of Southern California, Los Angeles, CA, 90089, USA. foxjuly@gmail.com.
Organizational Affiliation: