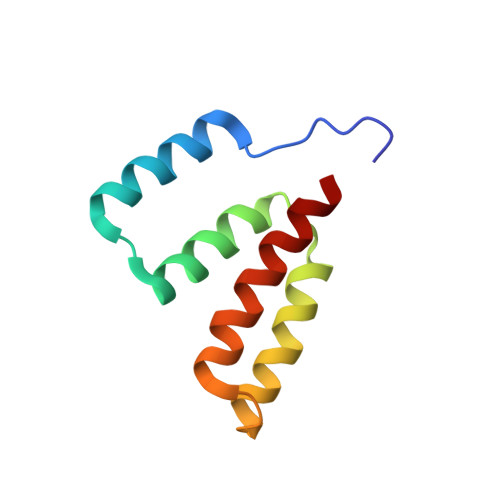
Identification of a conserved alpha-helical domain at the N terminus of human DNA methyltransferase 1.
Hu, Q., Botuyan, M.V., Mer, G.(2024) J Biological Chem 300: 105775-105775
- PubMed: 38382673
- DOI: https://doi.org/10.1016/j.jbc.2024.105775
- Primary Citation of Related Structures:
8V9U - PubMed Abstract:
In vertebrates, DNA methyltransferase 1 (DNMT1) contributes to preserving DNA methylation patterns, ensuring the stability and heritability of epigenetic marks important for gene expression regulation and the maintenance of cellular identity. Previous structural studies have elucidated the catalytic mechanism of DNMT1 and its specific recognition of hemimethylated DNA. Here, using solution nuclear magnetic resonance spectroscopy and small-angle X-ray scattering, we demonstrate that the N-terminal region of human DNMT1, while flexible, encompasses a conserved globular domain with a novel α-helical bundle-like fold. This work expands our understanding of the structure and dynamics of DNMT1 and provides a structural framework for future functional studies in relation with this new domain.
- Department of Biochemistry and Molecular Biology, Mayo Clinic, Rochester, Minnesota, USA.
Organizational Affiliation: