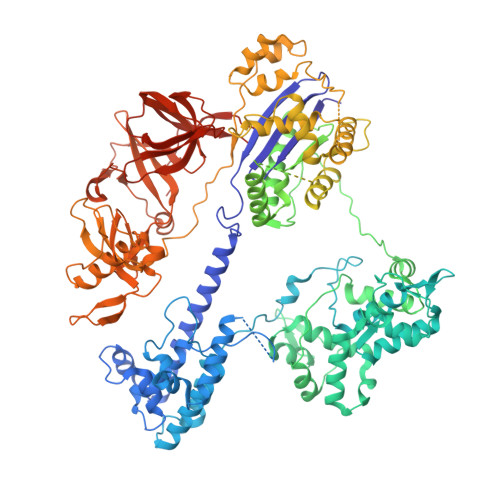
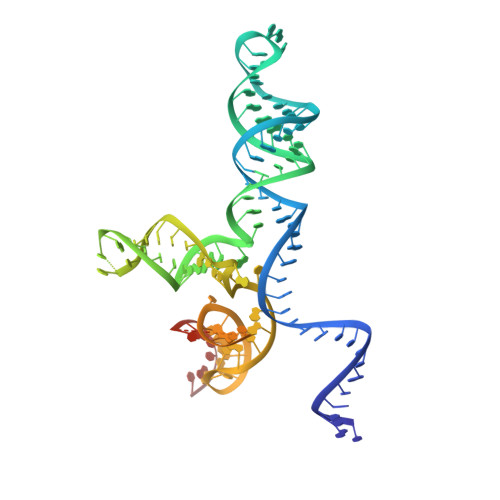
Rapid DNA unwinding accelerates genome editing by engineered CRISPR-Cas9.
Eggers, A.R., Chen, K., Soczek, K.M., Tuck, O.T., Doherty, E.E., Xu, B., Trinidad, M.I., Thornton, B.W., Yoon, P.H., Doudna, J.A.(2024) Cell 187: 3249
- PubMed: 38781968
- DOI: https://doi.org/10.1016/j.cell.2024.04.031
- Primary Citation of Related Structures:
8UZA, 8UZB - PubMed Abstract:
Thermostable clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated (Cas9) enzymes could improve genome-editing efficiency and delivery due to extended protein lifetimes. However, initial experimentation demonstrated Geobacillus stearothermophilus Cas9 (GeoCas9) to be virtually inactive when used in cultured human cells. Laboratory-evolved variants of GeoCas9 overcome this natural limitation by acquiring mutations in the wedge (WED) domain that produce >100-fold-higher genome-editing levels. Cryoelectron microscopy (cryo-EM) structures of the wild-type and improved GeoCas9 (iGeoCas9) enzymes reveal extended contacts between the WED domain of iGeoCas9 and DNA substrates. Biochemical analysis shows that iGeoCas9 accelerates DNA unwinding to capture substrates under the magnesium-restricted conditions typical of mammalian but not bacterial cells. These findings enabled rational engineering of other Cas9 orthologs to enhance genome-editing levels, pointing to a general strategy for editing enzyme improvement. Together, these results uncover a new role for the Cas9 WED domain in DNA unwinding and demonstrate how accelerated target unwinding dramatically improves Cas9-induced genome-editing activity.
- Department of Molecular and Cell Biology, University of California, Berkeley, Berkeley, CA 94720, USA; Innovative Genomics Institute, University of California, Berkeley, Berkeley, CA 94720, USA.
Organizational Affiliation: