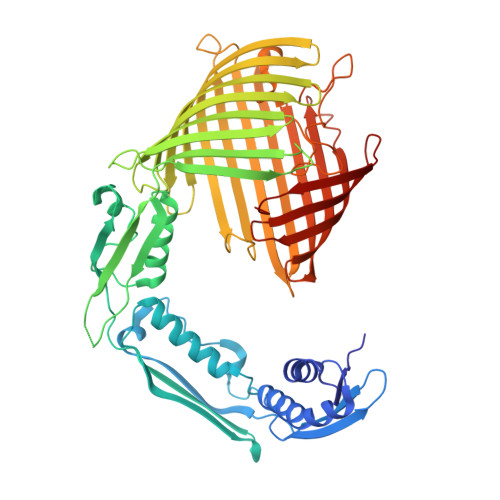
POTRA domains of the TamA insertase interact with the outer membrane and modulate membrane properties.
Mellouk, A., Jaouen, P., Ruel, L.J., Le, M., Martini, C., Moraes, T.F., El Bakkouri, M., Lague, P., Boisselier, E., Calmettes, C.(2024) Proc Natl Acad Sci U S A 121: e2402543121-e2402543121
- PubMed: 38959031
- DOI: https://doi.org/10.1073/pnas.2402543121
- Primary Citation of Related Structures:
8US1, 8US2, 8US3, 8US4 - PubMed Abstract:
The outer membrane (OM) of gram-negative bacteria serves as a vital organelle that is densely populated with OM proteins (OMPs) and plays pivotal roles in cellular functions and virulence. The assembly and insertion of these OMPs into the OM represent a fundamental process requiring specialized molecular chaperones. One example is the translocation and assembly module (TAM), which functions as a transenvelope chaperone promoting the folding of specific autotransporters, adhesins, and secretion systems. The catalytic unit of TAM, TamA, comprises a catalytic β-barrel domain anchored within the OM and three periplasmic polypeptide-transport-associated (POTRA) domains that recruit the TamB subunit. The latter acts as a periplasmic ladder that facilitates the transport of unfolded OMPs across the periplasm. In addition to their role in recruiting the auxiliary protein TamB, our data demonstrate that the POTRA domains mediate interactions with the inner surface of the OM, ultimately modulating the membrane properties. Through the integration of X-ray crystallography, molecular dynamic simulations, and biomolecular interaction methodologies, we located the membrane-binding site on the first and second POTRA domains. Our data highlight a binding preference for phosphatidylglycerol, a minor lipid constituent present in the OM, which has been previously reported to facilitate OMP assembly. In the context of the densely OMP-populated membrane, this association may serve as a mechanism to secure lipid accessibility for nascent OMPs through steric interactions with existing OMPs, in addition to creating favorable conditions for OMP biogenesis.
Organizational Affiliation:
Institut National de la Rechyuerche Scientifique (INRS), Centre Armand-Frappier Santé Biotechnologie, Laval, QC H7V 1B7, Canada.