Structural studies on the PU.1 inhibitory mechanism by diamidine minor groove binders
Terrell, J.R., Paul, A., Ogbonna, E.N., Farahat, A.A., Poon, G.M.K., Wilson, W.D.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Transcription factor PU.1 | 106 | Homo sapiens | Mutation(s): 0 Gene Names: SPI1 | 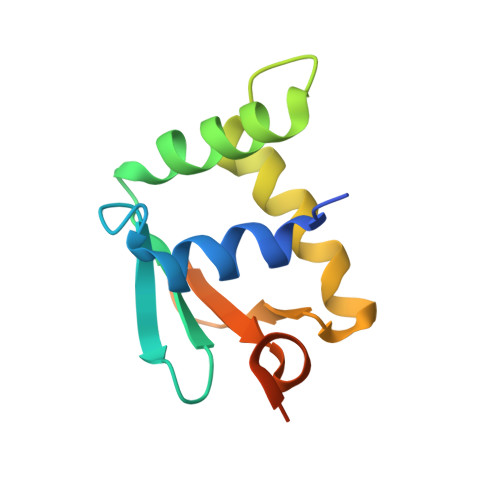 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P17947 (Homo sapiens) Explore P17947 Go to UniProtKB: P17947 | |||||
PHAROS: P17947 GTEx: ENSG00000066336 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P17947 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar nucleic acids by: Sequence | 3D Structure
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | Organism | Image | |
| DNA (5'-D(*AP*AP*AP*AP*TP*AP*AP*AP*AP*GP*GP*AP*AP*GP*TP*G)-3') | 16 | synthetic construct | 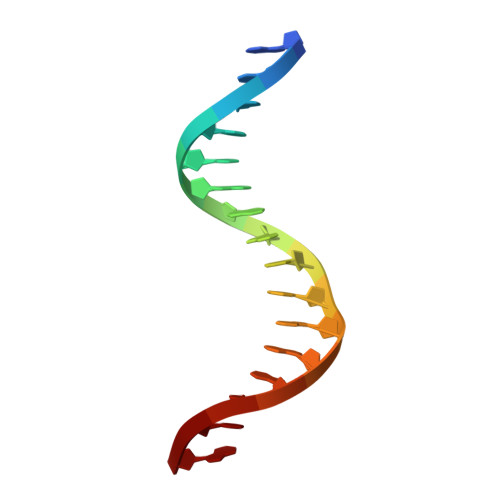 | ||
Sequence AnnotationsExpand | |||||
| |||||
Find similar nucleic acids by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | Organism | Image | |
| DNA (5'-D(*TP*CP*AP*CP*TP*TP*CP*CP*TP*TP*TP*TP*AP*TP*TP*T)-3') | 16 | synthetic construct | 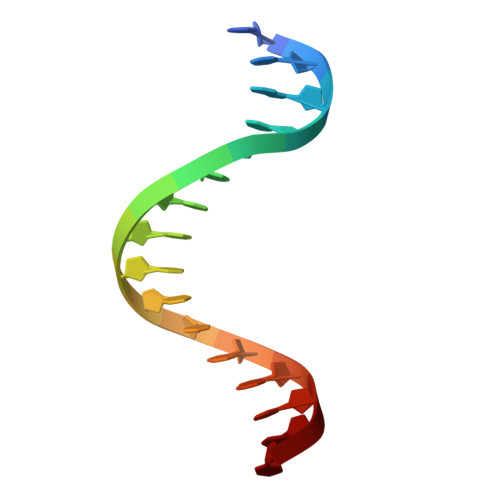 | ||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 91.211 | α = 90 |
| b = 100.013 | β = 106.961 |
| c = 54.875 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| XDS | data reduction |
| Aimless | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institutes of Health/National Heart, Lung, and Blood Institute (NIH/NHLBI) | United States | HL155178 |
| National Science Foundation (NSF, United States) | United States | MCB2028902 |
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | GM137160 |
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | GM111749 |