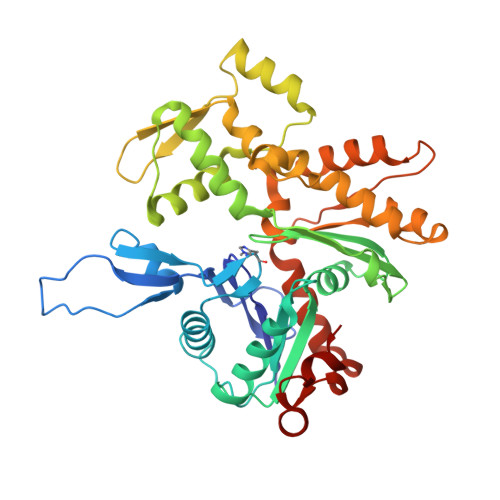
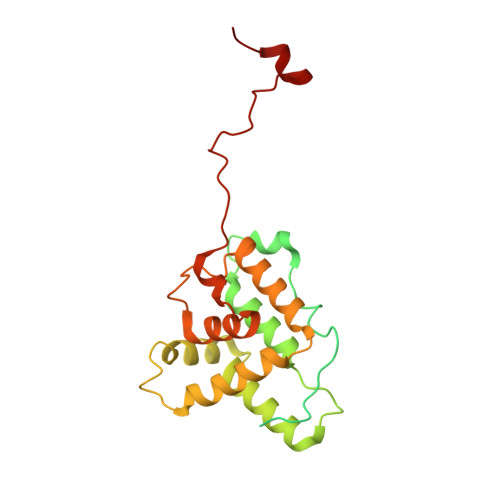
Stabilization of F-actin by Salmonella effector SipA resembles the structural effects of inorganic phosphate and phalloidin.
Niedzialkowska, E., Runyan, L.A., Kudryashova, E., Egelman, E.H., Kudryashov, D.S.(2024) Structure 32: 725-738.e8
- PubMed: 38518780
- DOI: https://doi.org/10.1016/j.str.2024.02.022
- Primary Citation of Related Structures:
8UEE - PubMed Abstract:
Entry of Salmonella into host enterocytes relies on its pathogenicity island 1 effector SipA. We found that SipA binds to F-actin in a 1:2 stoichiometry with sub-nanomolar affinity. A cryo-EM reconstruction revealed that SipA's globular core binds at the groove between actin strands, whereas the extended C-terminal arm penetrates deeply into the inter-strand space, stabilizing F-actin from within. The unusually strong binding of SipA is achieved by a combination of fast association via the core and very slow dissociation dictated by the arm. Similar to P i , BeF 3 , and phalloidin, SipA potently inhibited actin depolymerization by actin depolymerizing factor (ADF)/cofilin, which correlated with increased filament stiffness, supporting the hypothesis that F-actin's mechanical properties contribute to the recognition of its nucleotide state by protein partners. The remarkably strong binding to F-actin maximizes the toxin's effects at the injection site while minimizing global influence on the cytoskeleton and preventing pathogen detection by the host cell.
- Department of Biochemistry and Molecular Genetics, University of Virginia, Charlottesville, VA 22903, USA.
Organizational Affiliation: