Two-mode inhibition of DsbA: combination of two site-specific inhibitors enhances virulence inhibition in Salmonella enterica serovar Typhimurium
Wang, G., Heras, B.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Thiol:disulfide interchange protein DsbA | 189 | Escherichia coli K-12 | Mutation(s): 0 Gene Names: dsbA, dsf, ppfA, b3860, JW3832 | 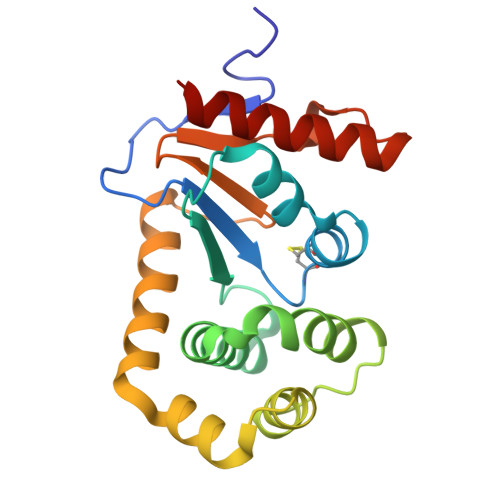 | |
UniProt | |||||
Find proteins for P0AEG4 (Escherichia coli (strain K12)) Explore P0AEG4 Go to UniProtKB: P0AEG4 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P0AEG4 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| W9H (Subject of Investigation/LOI) Query on W9H | C [auth A] | 2-benzyl-4-phenyl-1,3-thiazole-5-carboxylic acid C17 H13 N O2 S MKDPSENJELZGTK-UHFFFAOYSA-N |  | ||
| SW0 Query on SW0 | E [auth B] | N-(2-fluorophenyl)-5-methyl-1,2-oxazole-3-carboxamide C11 H9 F N2 O2 VZLZHRVTOSUIPW-UHFFFAOYSA-N |  | ||
| CU Query on CU | D [auth B] | COPPER (II) ION Cu JPVYNHNXODAKFH-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 115.971 | α = 90 |
| b = 64.76 | β = 125.93 |
| c = 74.842 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| Aimless | data scaling |
| PHASER | phasing |
| XDS | data reduction |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Health and Medical Research Council (NHMRC, Australia) | Australia | GNT1099151 |