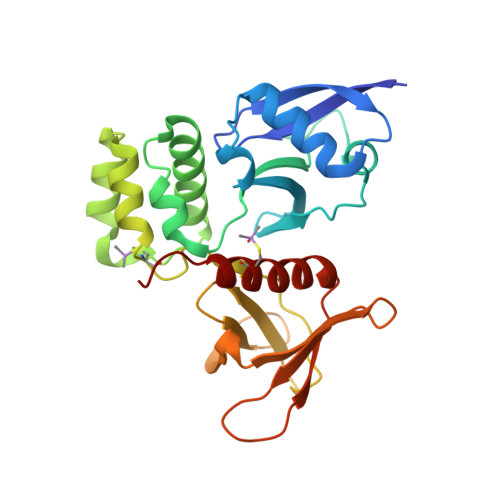
Structural basis for coupling of the WASH subunit FAM21 with the endosomal SNX27-Retromer complex.
Guo, Q., Chen, K.E., Gimenez-Andres, M., Jellett, A.P., Gao, Y., Simonetti, B., Liu, M., Danson, C.M., Heesom, K.J., Cullen, P.J., Collins, B.M.(2024) Proc Natl Acad Sci U S A 121: e2405041121-e2405041121
- PubMed: 39116126
- DOI: https://doi.org/10.1073/pnas.2405041121
- Primary Citation of Related Structures:
8TTA, 8TTC, 8TTD, 8TTT, 8TTU, 8TTV - PubMed Abstract:
Endosomal membrane trafficking is mediated by specific protein coats and formation of actin-rich membrane domains. The Retromer complex coordinates with sorting nexin (SNX) cargo adaptors including SNX27, and the SNX27-Retromer assembly interacts with the Wiskott-Aldrich syndrome protein and SCAR homolog (WASH) complex which nucleates actin filaments establishing the endosomal recycling domain. Crystal structures, modeling, biochemical, and cellular validation reveal how the FAM21 subunit of WASH interacts with both Retromer and SNX27. FAM21 binds the FERM domain of SNX27 using acidic-Asp-Leu-Phe (aDLF) motifs similar to those found in the SNX1 and SNX2 subunits of the ESCPE-1 complex. Overlapping FAM21 repeats and a specific Pro-Leu containing motif bind three distinct sites on Retromer involving both the VPS35 and VPS29 subunits. Mutation of the major VPS35-binding site does not prevent cargo recycling; however, it partially reduces endosomal WASH association indicating that a network of redundant interactions promote endosomal activity of the WASH complex. These studies establish the molecular basis for how SNX27-Retromer is coupled to the WASH complex via overlapping and multiplexed motif-based interactions required for the dynamic assembly of endosomal membrane recycling domains.
- The University of Queensland, Institute for Molecular Bioscience, St Lucia, QLD 4072, Australia.
Organizational Affiliation: