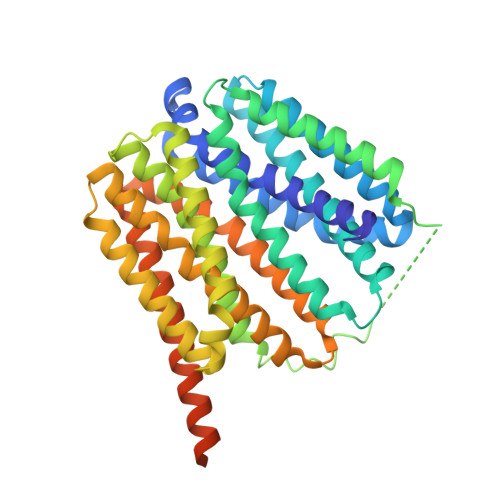
Proton-coupled transport mechanism of the efflux pump NorA.
Li, J., Li, Y., Koide, A., Kuang, H., Torres, V.J., Koide, S., Wang, D.N., Traaseth, N.J.(2024) Nat Commun 15: 4494-4494
- PubMed: 38802368
- DOI: https://doi.org/10.1038/s41467-024-48759-3
- Primary Citation of Related Structures:
8TTE, 8TTF, 8TTG, 8TTH - PubMed Abstract:
Efflux pump antiporters confer drug resistance to bacteria by coupling proton import with the expulsion of antibiotics from the cytoplasm. Despite efforts there remains a lack of understanding as to how acid/base chemistry drives drug efflux. Here, we uncover the proton-coupling mechanism of the Staphylococcus aureus efflux pump NorA by elucidating structures in various protonation states of two essential acidic residues using cryo-EM. Protonation of Glu222 and Asp307 within the C-terminal domain stabilized the inward-occluded conformation by forming hydrogen bonds between the acidic residues and a single helix within the N-terminal domain responsible for occluding the substrate binding pocket. Remarkably, deprotonation of both Glu222 and Asp307 is needed to release interdomain tethering interactions, leading to opening of the pocket for antibiotic entry. Hence, the two acidic residues serve as a "belt and suspenders" protection mechanism to prevent simultaneous binding of protons and drug that enforce NorA coupling stoichiometry and confer antibiotic resistance.
- Department of Chemistry, New York University, New York, NY, USA.
Organizational Affiliation: