Broad Neutralizing Antibodies Against Coronaviruses.
Feng, Z., Wilson, I.A.Not Published
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Neutralizing antibody CHM-27 Heavy Chain | A [auth H] | 217 | Macaca mulatta | Mutation(s): 0 | 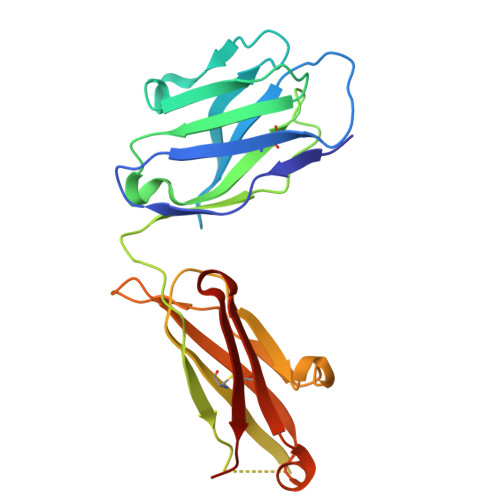 |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Neutralizing antibody CHM-27 Light Chain | B [auth L] | 216 | Macaca mulatta | Mutation(s): 0 | 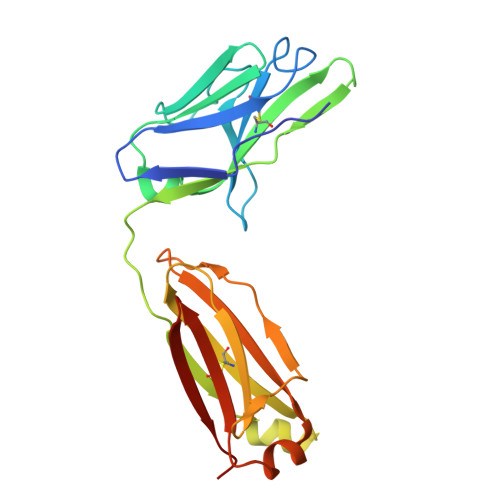 |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Stem helix peptide of Spike glycoprotein | C [auth A] | 27 | Middle East respiratory syndrome-related coronavirus | Mutation(s): 0 | 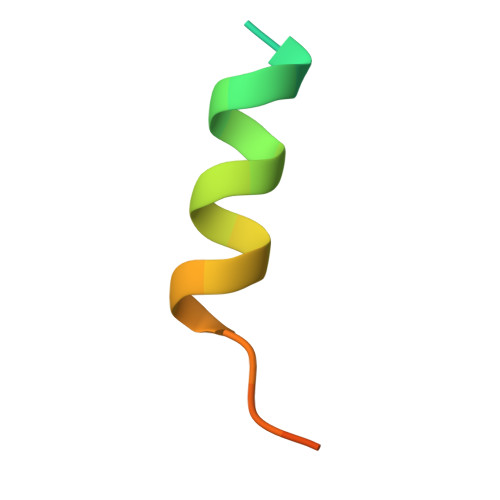 |
UniProt | |||||
Find proteins for K9N5Q8 (Middle East respiratory syndrome-related coronavirus (isolate United Kingdom/H123990006/2012)) Explore K9N5Q8 Go to UniProtKB: K9N5Q8 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | K9N5Q8 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| EDO Query on EDO | D [auth L] | 1,2-ETHANEDIOL C2 H6 O2 LYCAIKOWRPUZTN-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 43.057 | α = 90 |
| b = 71.402 | β = 106 |
| c = 74.571 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| autoPROC | data reduction |
| autoPROC | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Bill & Melinda Gates Foundation | United States | INV-004923 |