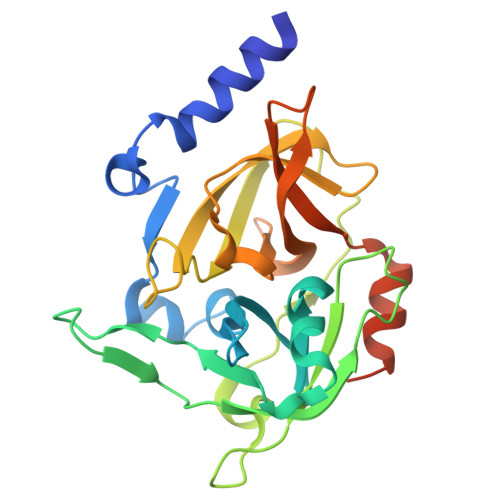
Necrotic activity of ExhC from Mammaliicoccus sciuri is mediated by specific amino acid residues.
Gismene, C., Gonzalez, J.E.H., de Freitas Calmon, M., Nascimento, A.F.Z., Santisteban, A.R.N., Calil, F.A., da Silva, A.D.T., Rahal, P., Goes, R.M., Arni, R.K., Mariutti, R.B.(2024) Int J Biol Macromol 254: 127741-127741
- PubMed: 38287568
- DOI: https://doi.org/10.1016/j.ijbiomac.2023.127741
- Primary Citation of Related Structures:
8T3I, 8T3J - PubMed Abstract:
Mammaliicoccus sciuri, a commensal and pathogenic bacterium of significant clinical and veterinary relevance, expresses exfoliative toxin C (ExhC), a specific glutamyl endopeptidase belonging to the chymotrypsin family as the principal virulence factor. However, unlike most members of this family, ETs are inactive against a wide range of substrates and possess exquisite specificity for desmoglein-1 (Dsg1), a cadherin-like adhesion molecule that is crucial to maintain tissue integrity, thereby preventing the separation of skin cells and the entry of pathogens. ExhC is of clinical importance since in addition to causing exfoliation in pigs and mice, it induces necrosis in multiple mammalian cell lines, a property not observed for other ETs. Previous experiments have implicated the ExhC 79 - 128 fragment in causing necrosis. Site-directed mutagenesis of specific residues within this fragment were studied and led to the design of an ExhC variant containing four-point mutations (ExhC mut4 ) lacking necrotic potential but retaining nearly wild-type (wt) levels of enzymatic activity. Moreover, the determination of the ExhC wt and ExhC mut4 crystal structures identified the conformation in the necrosis-linked region. These results constitute an important step toward the understanding of the mechanisms underlying the necrotic and epidermolytic activity of ExhC.
- Multiuser Center for Biomolecular Innovation, São Paulo State University - UNESP, São José do Rio Preto, SP, Brazil.
Organizational Affiliation: