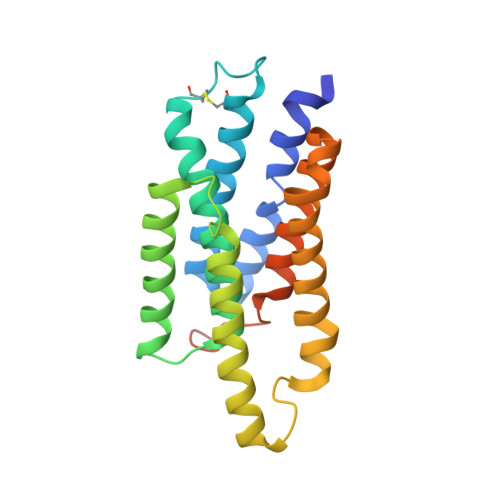
Cryo-EM structures of Myomaker reveal a molecular basis for myoblast fusion.
Long, T., Zhang, Y., Donnelly, L., Li, H., Pien, Y.C., Liu, N., Olson, E.N., Li, X.(2023) Nat Struct Mol Biol 30: 1746-1754
- PubMed: 37770716
- DOI: https://doi.org/10.1038/s41594-023-01110-8
- Primary Citation of Related Structures:
8T03, 8T04, 8T05, 8T06, 8T07 - PubMed Abstract:
The fusion of mononucleated myoblasts produces multinucleated muscle fibers leading to the formation of skeletal muscle. Myomaker, a skeletal muscle-specific membrane protein, is essential for myoblast fusion. Here we report the cryo-EM structures of mouse Myomaker (mMymk) and Ciona robusta Myomaker (cMymk). Myomaker contains seven transmembrane helices (TMs) that adopt a G-protein-coupled receptor-like fold. TMs 2-4 form a dimeric interface, while TMs 3 and 5-7 create a lipid-binding site that holds the polar head of a phospholipid and allows the alkyl tails to insert into Myomaker. The similarity of cMymk and mMymk suggests a conserved Myomaker-mediated cell fusion mechanism across evolutionarily distant species. Functional analyses demonstrate the essentiality of the dimeric interface and the lipid-binding site for fusogenic activity, and heterologous cell-cell fusion assays show the importance of transcellular interactions of Myomaker protomers for myoblast fusion. Together, our findings provide structural and functional insights into the process of myoblast fusion.
- Department of Molecular Genetics, University of Texas Southwestern Medical Center, Dallas, TX, USA.
Organizational Affiliation: