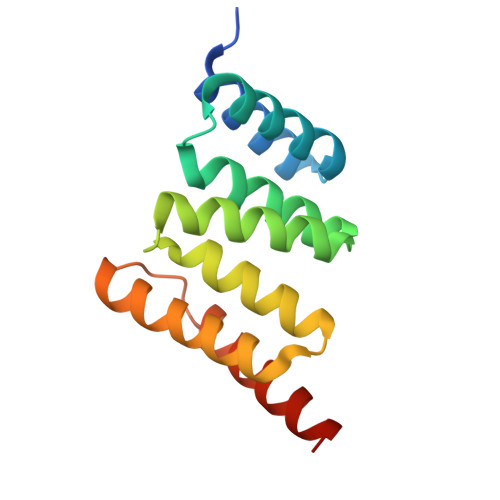
Interaction with the membrane-anchored protein CHIC2 constrains the ubiquitin ligase activity of CHIP
Callahan, M., Hodul, M., Carroll, E., Ravalin, M., Nadel, C., de Silva, A., Cupo, A., McDermott, L., Nix, J., Page, R., Kao, A., Gestwicki, J.(2023) bioRxiv