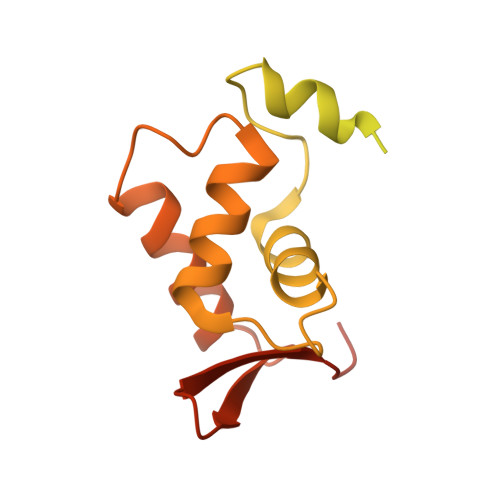
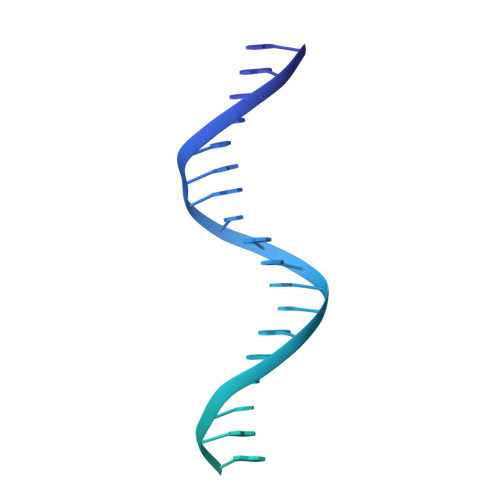
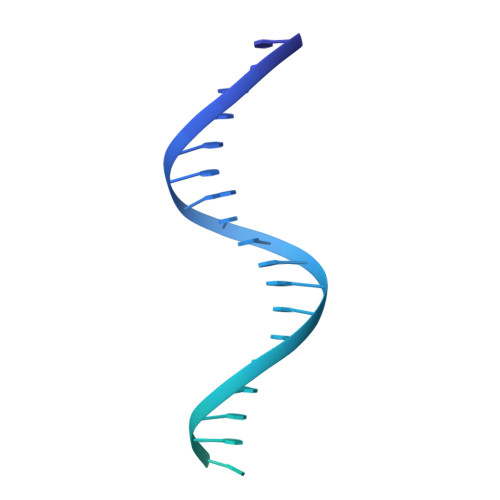
FOXP3 recognizes microsatellites and bridges DNA through multimerization.
Zhang, W., Leng, F., Wang, X., Ramirez, R.N., Park, J., Benoist, C., Hur, S.(2023) Nature 624: 433-441
- PubMed: 38030726
- DOI: https://doi.org/10.1038/s41586-023-06793-z
- Primary Citation of Related Structures:
8SRO, 8SRP - PubMed Abstract:
FOXP3 is a transcription factor that is essential for the development of regulatory T cells, a branch of T cells that suppress excessive inflammation and autoimmunity 1-5 . However, the molecular mechanisms of FOXP3 remain unclear. Here we here show that FOXP3 uses the forkhead domain-a DNA-binding domain that is commonly thought to function as a monomer or dimer-to form a higher-order multimer after binding to T n G repeat microsatellites. The cryo-electron microscopy structure of FOXP3 in a complex with T 3 G repeats reveals a ladder-like architecture, whereby two double-stranded DNA molecules form the two 'side rails' bridged by five pairs of FOXP3 molecules, with each pair forming a 'rung'. Each FOXP3 subunit occupies TGTTTGT within the repeats in a manner that is indistinguishable from that of FOXP3 bound to the forkhead consensus motif (TGTTTAC). Mutations in the intra-rung interface impair T n G repeat recognition, DNA bridging and the cellular functions of FOXP3, all without affecting binding to the forkhead consensus motif. FOXP3 can tolerate variable inter-rung spacings, explaining its broad specificity for T n G-repeat-like sequences in vivo and in vitro. Both FOXP3 orthologues and paralogues show similar T n G repeat recognition and DNA bridging. These findings therefore reveal a mode of DNA recognition that involves transcription factor homomultimerization and DNA bridging, and further implicates microsatellites in transcriptional regulation and diseases.
- Howard Hughes Medical Institute and Program in Cellular and Molecular Medicine, Boston Children's Hospital, Boston, MA, USA.
Organizational Affiliation: