Coupling enzymatic activity and gating in an ancient TRPM chanzyme and its molecular evolution.
Huang, Y., Kumar, S., Lee, J., Lu, W., Du, J.(2024) Nat Struct Mol Biol 31: 1509-1521
- PubMed: 38773335
- DOI: https://doi.org/10.1038/s41594-024-01316-4
- Primary Citation of Related Structures:
8SR7, 8SR8, 8SR9, 8SRA, 8SRB, 8SRC, 8SRD, 8SRE, 8SRF, 8SRG, 8SRH, 8SRI, 8SRJ, 8SRK - PubMed Abstract:
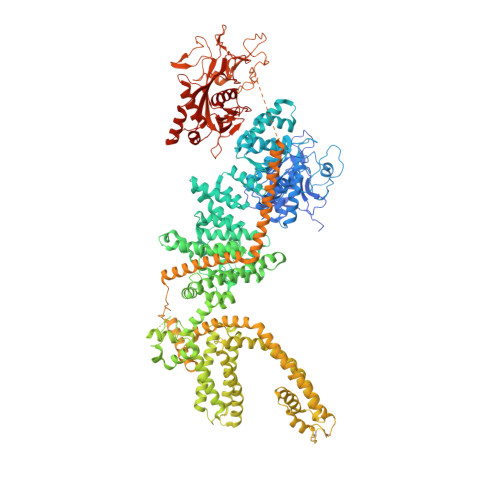
Channel enzymes represent a class of ion channels with enzymatic activity directly or indirectly linked to their channel function. We investigated a TRPM2 chanzyme from choanoflagellates that integrates two seemingly incompatible functions into a single peptide: a channel module activated by ADP-ribose with high open probability and an enzyme module (NUDT9-H domain) consuming ADP-ribose at a remarkably slow rate. Using time-resolved cryogenic-electron microscopy, we captured a complete series of structural snapshots of gating and catalytic cycles, revealing the coupling mechanism between channel gating and enzymatic activity. The slow kinetics of the NUDT9-H enzyme module confers a self-regulatory mechanism: ADPR binding triggers NUDT9-H tetramerization, promoting channel opening, while subsequent hydrolysis reduces local ADPR, inducing channel closure. We further demonstrated how the NUDT9-H domain has evolved from a structurally semi-independent ADP-ribose hydrolase module in early species to a fully integrated component of a gating ring essential for channel activation in advanced species.
- Van Andel Institute, Grand Rapids, MI, USA.
Organizational Affiliation: