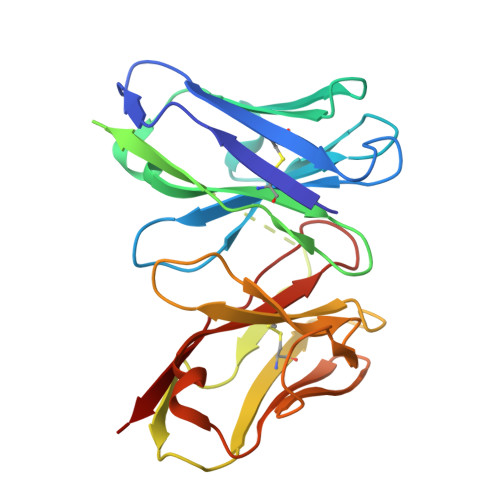
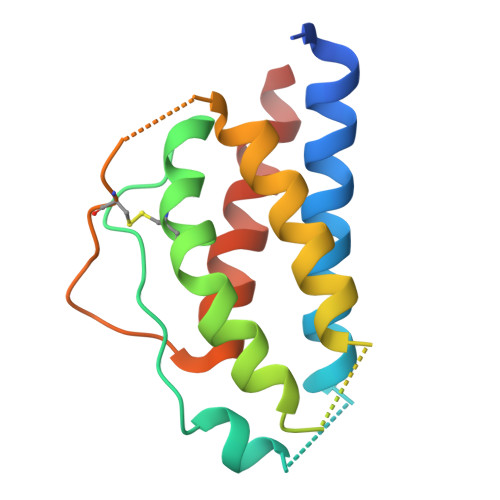
Engineered cytokine/antibody fusion proteins improve IL-2 delivery to pro-inflammatory cells and promote antitumor activity.
Leonard, E.K., Tomala, J., Gould, J.R., Leff, M.I., Lin, J.X., Li, P., Porter, M.J., Johansen, E.R., Thompson, L., Cao, S.D., Hou, S., Henclova, T., Huliciak, M., Sargunas, P.R., Fabilane, C.S., Vanek, O., Kovar, M., Schneider, B., Raimondi, G., Leonard, W.J., Spangler, J.B.(2024) JCI Insight 9
- PubMed: 39115939
- DOI: https://doi.org/10.1172/jci.insight.173469
- Primary Citation of Related Structures:
8SOW, 8SOZ - PubMed Abstract:
Progress in cytokine engineering is driving therapeutic translation by overcoming these proteins' limitations as drugs. The interleukin-2 (IL-2) cytokine is a promising immune stimulant for cancer treatment but is limited by its concurrent activation of both pro-inflammatory immune effector cells and anti-inflammatory regulatory T cells, toxicity at high doses, and short serum half-life. One approach to improve the selectivity, safety, and longevity of IL-2 is complexation with anti-IL-2 antibodies that bias the cytokine towards immune effector cell activation. Although this strategy shows potential in preclinical models, clinical translation of a cytokine/antibody complex is complicated by challenges in formulating a multi-protein drug and concerns regarding complex stability. Here, we introduced a versatile approach to designing intramolecularly assembled single-agent fusion proteins (immunocytokines, ICs) comprising IL-2 and a biasing anti-IL-2 antibody that directs the cytokine towards immune effector cells. We optimized IC construction and engineered the cytokine/antibody affinity to improve immune bias. We demonstrated that our IC preferentially activates and expands immune effector cells, leading to superior antitumor activity compared to natural IL-2, both alone and combined with immune checkpoint inhibitors. Moreover, therapeutic efficacy was observed without inducing toxicity. This work presents a roadmap for the design and translation of cytokine/antibody fusion proteins.
- Biomedical Engineering, Johns Hopkins University School of Medicine, Baltimore, United States of America.
Organizational Affiliation: