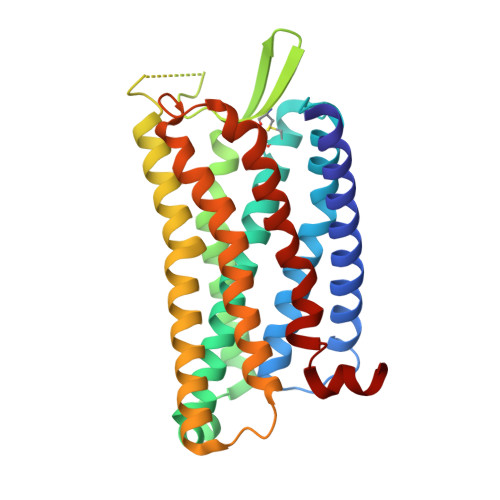
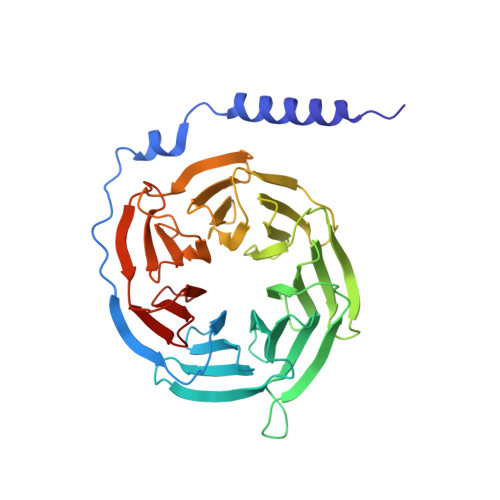
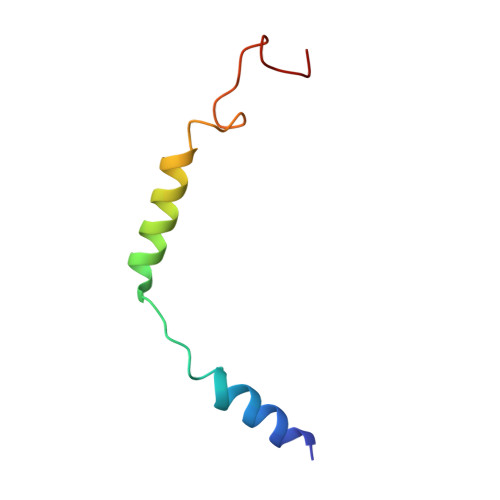
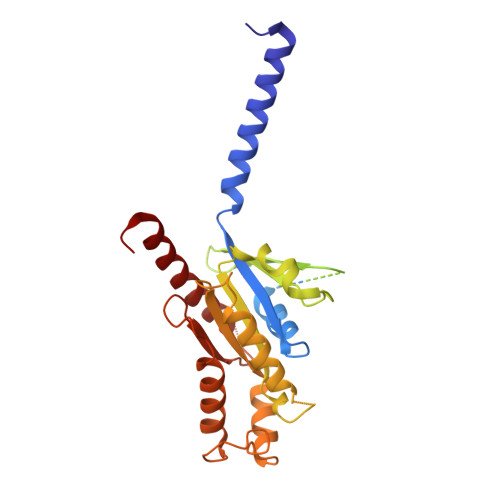
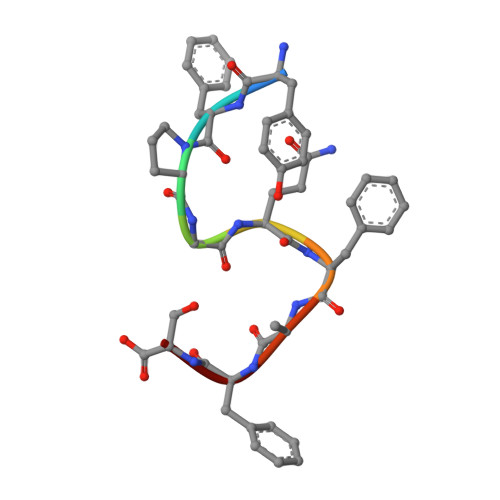
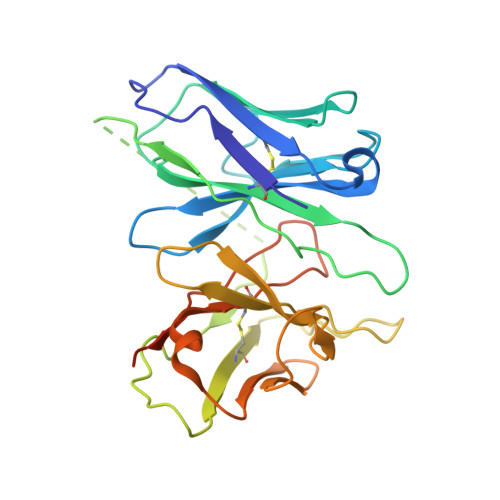
Structural basis of G protein-Coupled receptor CMKLR1 activation and signaling induced by a chemerin-derived agonist.
Zhang, X., Weiss, T., Cheng, M.H., Chen, S., Ambrosius, C.K., Czerniak, A.S., Li, K., Feng, M., Bahar, I., Beck-Sickinger, A.G., Zhang, C.(2023) PLoS Biol 21: e3002188-e3002188
- PubMed: 38055679
- DOI: https://doi.org/10.1371/journal.pbio.3002188
- Primary Citation of Related Structures:
8SG1 - PubMed Abstract:
Chemokine-like receptor 1 (CMKLR1), also known as chemerin receptor 23 (ChemR23) or chemerin receptor 1, is a chemoattractant G protein-coupled receptor (GPCR) that responds to the adipokine chemerin and is highly expressed in innate immune cells, including macrophages and neutrophils. The signaling pathways of CMKLR1 can lead to both pro- and anti-inflammatory effects depending on the ligands and physiological contexts. To understand the molecular mechanisms of CMKLR1 signaling, we determined a high-resolution cryo-electron microscopy (cryo-EM) structure of the CMKLR1-Gi signaling complex with chemerin9, a nanopeptide agonist derived from chemerin, which induced complex phenotypic changes of macrophages in our assays. The cryo-EM structure, together with molecular dynamics simulations and mutagenesis studies, revealed the molecular basis of CMKLR1 signaling by elucidating the interactions at the ligand-binding pocket and the agonist-induced conformational changes. Our results are expected to facilitate the development of small molecule CMKLR1 agonists that mimic the action of chemerin9 to promote the resolution of inflammation.
- Department of Pharmacology and Chemical Biology, School of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, United States of America.
Organizational Affiliation: