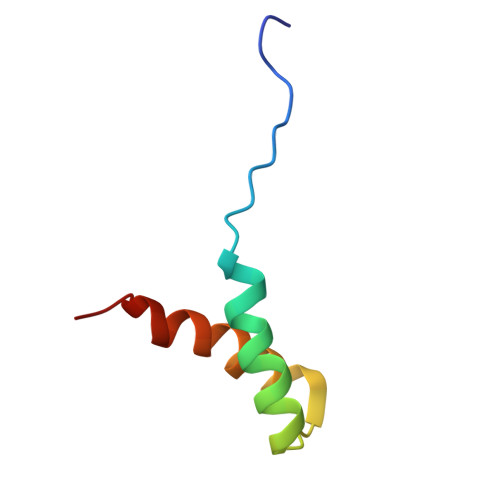
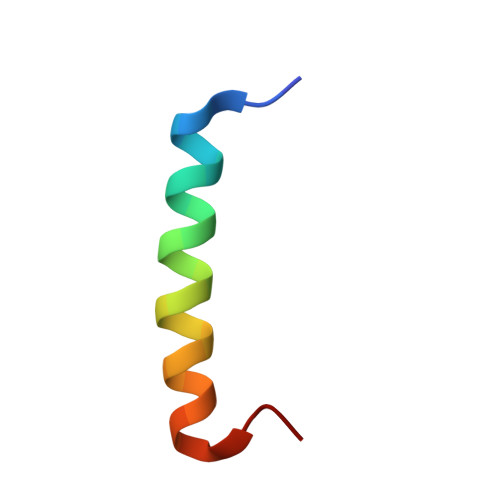
Structural basis of binding the unique N-terminal domain of microtubule-associated protein 2c to proteins regulating kinases of signaling pathways.
Bartosik, V., Plucarova, J., Lanikova, A., Janackova, Z., Padrta, P., Jansen, S., Varecka, V., Gruber, T., Feller, S.M., Zidek, L.(2024) J Biological Chem 300: 107551-107551
- PubMed: 39002671
- DOI: https://doi.org/10.1016/j.jbc.2024.107551
- Primary Citation of Related Structures:
8S8O - PubMed Abstract:
Isoforms of microtubule-associated protein 2 (MAP2) differ from their homolog Tau in the sequence and interactions of the N-terminal region. Binding of the N-terminal region of MAP2c (N-MAP2c) to the dimerization/docking domains of the regulatory subunit RIIα of cAMP-dependent protein kinase (RIIDD 2 ) and to the Src-homology domain 2 (SH2) of growth factor receptor-bound protein 2 (Grb2) have been described long time ago. However, the structural features of the complexes remained unknown due to the disordered nature of MAP2. Here, we provide structural description of the complexes. We have solved solution structure of N-MAP2c in complex with RIIDD 2 , confirming formation of an amphiphilic α-helix of MAP2c upon binding, defining orientation of the α-helix in the complex and showing that its binding register differs from previous predictions. Using chemical shift mapping, we characterized the binding interface of SH2-Grb2 and rat MAP2c phosphorylated by the tyrosine kinase Fyn in their complex and proposed a model explaining differences between SH2-Grb2 complexes with rat MAP2c and phosphopeptides with a Grb2-specific sequence. The results provide the structural basis of a potential role of MAP2 in regulating cAMP-dependent phosphorylation cascade via interactions with RIIDD 2 and Ras signaling pathway via interactions with SH2-Grb2.
- National Centre for Biomolecular Research, Faculty of Science, Masaryk University, Brno, Czech Republic.
Organizational Affiliation: