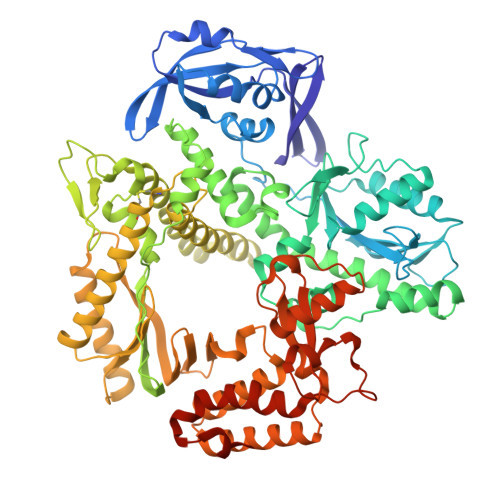
Structural insights into a DNA polymerase reading the xeno nucleic acid HNA.
Gutfreund, C., Betz, K., Abramov, M., Coosemans, F., Holliger, P., Herdewijn, P., Marx, A.(2025) Nucleic Acids Res 53
- PubMed: 39673482
- DOI: https://doi.org/10.1093/nar/gkae1156
- Primary Citation of Related Structures:
8S84, 8S87, 9EMI - PubMed Abstract:
Xeno nucleic acids (XNAs) are unnatural analogues of the natural nucleic acids in which the canonical ribose or deoxyribose rings are replaced with alternative sugars, congener structures or even open-ring configurations. The expanding repertoire of XNAs holds significant promise for diverse applications in molecular biology as well as diagnostics and therapeutics. Key advantages of XNAs over natural nucleic acids include their enhanced biostability, superior target affinity and (in some cases) catalytic activity. Natural systems generally lack the mechanisms to transcribe, reverse transcribe or replicate XNAs. This limitation has been overcome through the directed evolution of nucleic acid-modifying enzymes, especially polymerases (pols) and reverse transcriptases (RTs). Despite these advances, the mechanisms by which synthetic RT enzymes read these artificial genetic polymers remain largely unexplored, primarily due to a scarcity of structural information. This study unveils first structural insights into an evolved thermostable DNA pol interacting with the XNA 1,5-anhydrohexitol nucleic acid (HNA), revealing unprecedented HNA nucleotide conformations within a ternary complex with the enzyme. These findings not only deepen our understanding of HNA to DNA reverse transcription but also set the stage for future advancements of this and similar enzymes through deliberate design.
- Department of Chemistry, University of Konstanz, Universitätsstraße 10, 78457 Konstanz, Germany.
Organizational Affiliation: