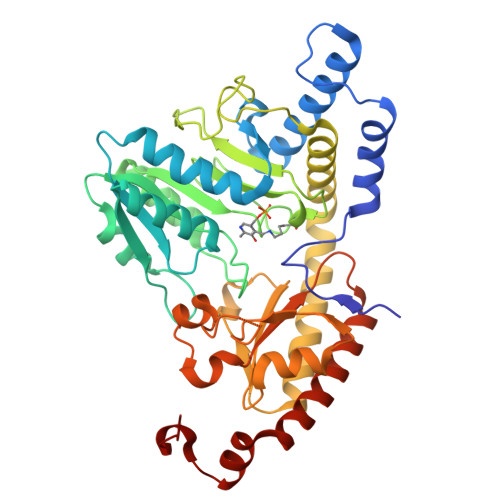
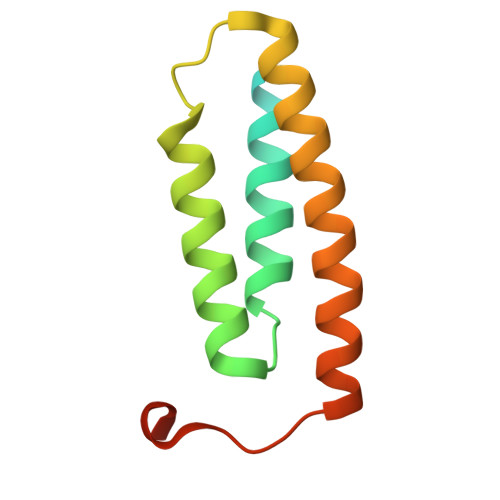
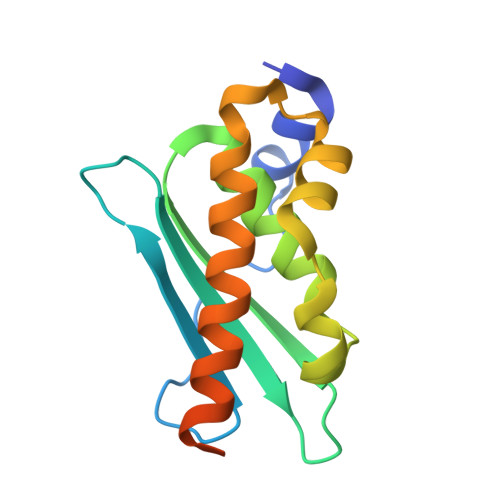
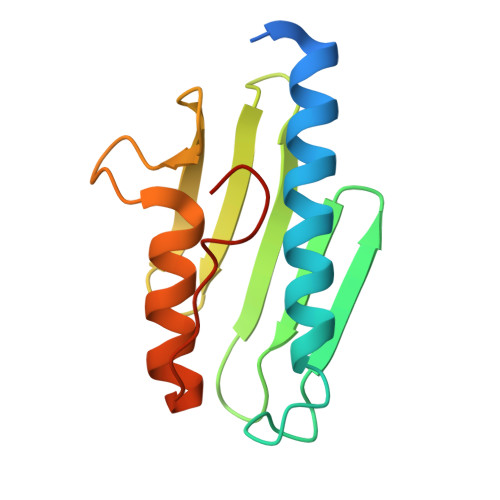
Two-stage binding of mitochondrial ferredoxin-2 to the core iron-sulfur cluster assembly complex.
Steinhilper, R., Boss, L., Freibert, S.A., Schulz, V., Krapoth, N., Kaltwasser, S., Lill, R., Murphy, B.J.(2024) Nat Commun 15: 10559-10559
- PubMed: 39632806
- DOI: https://doi.org/10.1038/s41467-024-54585-4
- Primary Citation of Related Structures:
8RMC, 8RMD, 8RME, 8RMF, 8RMG - PubMed Abstract:
Iron-sulfur (FeS) protein biogenesis in eukaryotes begins with the de novo assembly of [2Fe-2S] clusters by the mitochondrial core iron-sulfur cluster assembly (ISC) complex. This complex comprises the scaffold protein ISCU2, the cysteine desulfurase subcomplex NFS1-ISD11-ACP1, the allosteric activator frataxin (FXN) and the electron donor ferredoxin-2 (FDX2). The structural interaction of FDX2 with the complex remains unclear. Here, we present cryo-EM structures of the human FDX2-bound core ISC complex showing that FDX2 and FXN compete for overlapping binding sites. FDX2 binds in either a 'distal' conformation, where its helix F interacts electrostatically with an arginine patch of NFS1, or a 'proximal' conformation, where this interaction tightens and the FDX2-specific C terminus binds to NFS1, facilitating the movement of the [2Fe-2S] cluster of FDX2 closer to the ISCU2 FeS cluster assembly site for rapid electron transfer. Structure-based mutational studies verify the contact areas of FDX2 within the core ISC complex.
- Redox and Metalloprotein Research Group, Max Planck Institute of Biophysics, Max-von-Laue-Str. 3, 60438, Frankfurt am Main, Germany.
Organizational Affiliation: