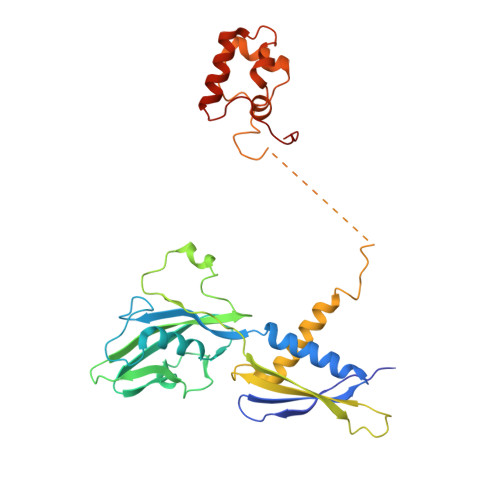
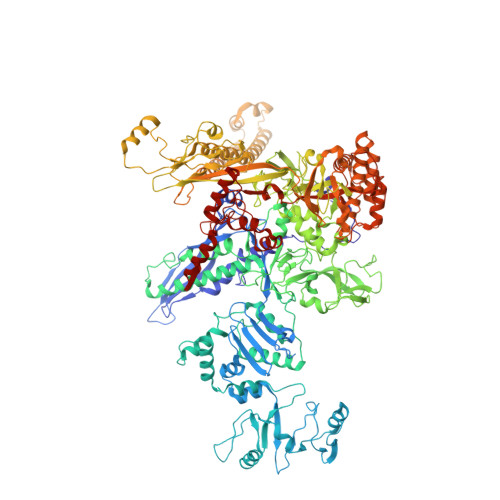
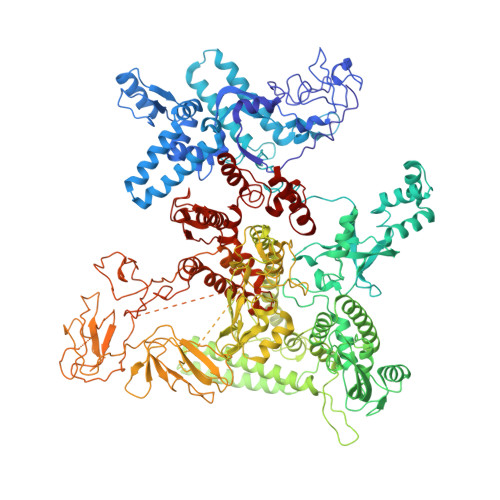
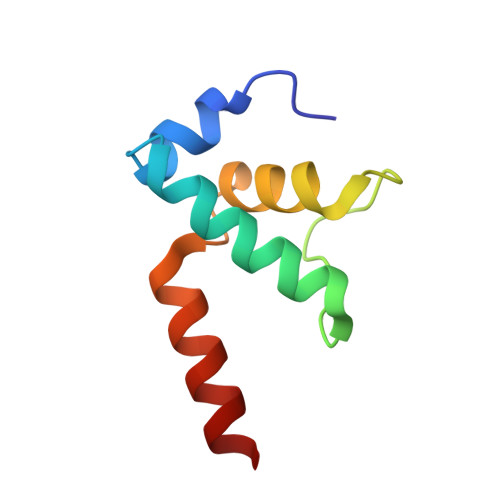
Structural basis of sigma 54 displacement and promoter escape in bacterial transcription.
Gao, F., Ye, F., Zhang, B., Cronin, N., Buck, M., Zhang, X.(2024) Proc Natl Acad Sci U S A 121: e2309670120-e2309670120
- PubMed: 38170755
- DOI: https://doi.org/10.1073/pnas.2309670120
- Primary Citation of Related Structures:
8RE4, 8REA, 8REB, 8REC, 8RED, 8REE - PubMed Abstract:
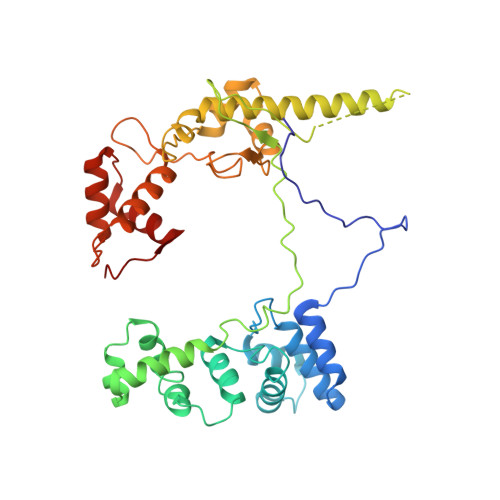
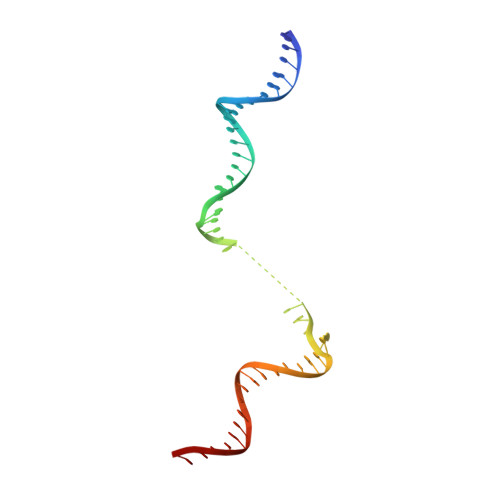
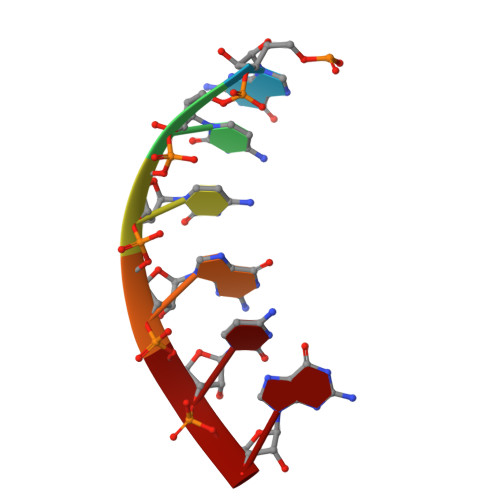
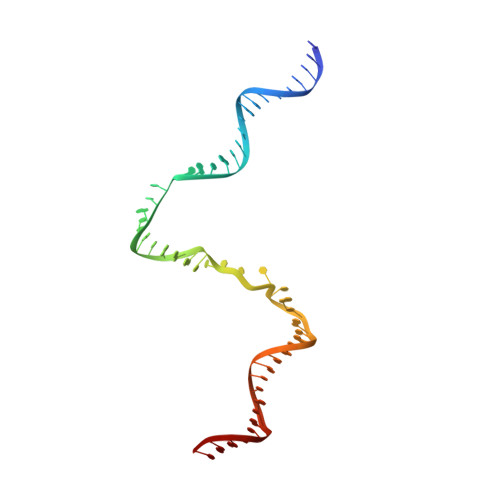
Gene transcription is a fundamental cellular process carried out by RNA polymerase (RNAP). Transcription initiation is highly regulated, and in bacteria, transcription initiation is mediated by sigma (σ) factors. σ recruits RNAP to the promoter DNA region, located upstream of the transcription start site (TSS) and facilitates open complex formation, where double-stranded DNA is opened up into a transcription bubble and template strand DNA is positioned inside RNAP for initial RNA synthesis. During initial transcription, RNAP remains bound to σ and upstream DNA, presumably with an enlarging transcription bubble. The release of RNAP from upstream DNA is required for promoter escape and processive transcription elongation. Bacteria sigma factors can be broadly separated into two classes with the majority belonging to the σ 70 class, represented by the σ 70 that regulates housekeeping genes. σ 54 forms a class on its own and regulates stress response genes. Extensive studies on σ 70 have revealed the molecular mechanisms of the σ 70 dependent process while how σ 54 transitions from initial transcription to elongation is currently unknown. Here, we present a series of cryo-electron microscopy structures of the RNAP-σ 54 initial transcribing complexes with progressively longer RNA, which reveal structural changes that lead to promoter escape. Our data show that initially, the transcription bubble enlarges, DNA strands scrunch, reducing the interactions between σ 54 and DNA strands in the transcription bubble. RNA extension and further DNA scrunching help to release RNAP from σ 54 and upstream DNA, enabling the transition to elongation.
- Section of Structural and Synthetic Biology, Department of Infectious Disease, Imperial College London, London SW7 2AZ, United Kingdom.
Organizational Affiliation: