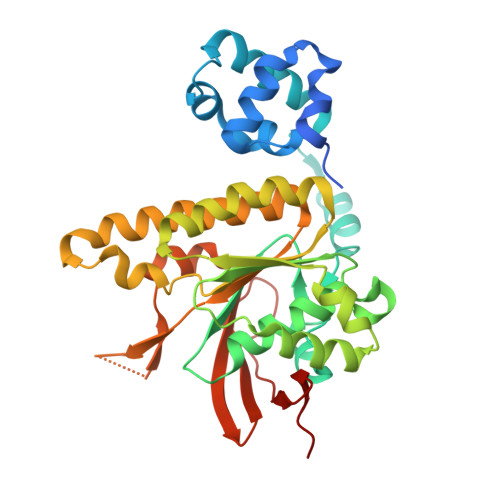
RAD51 protects abasic sites to prevent replication fork breakage.
Hanthi, Y.W., Ramirez-Otero, M.A., Appleby, R., De Antoni, A., Joudeh, L., Sannino, V., Waked, S., Ardizzoia, A., Barra, V., Fachinetti, D., Pellegrini, L., Costanzo, V.(2024) Mol Cell 84: 3026-3043.e11
- PubMed: 39178838
- DOI: https://doi.org/10.1016/j.molcel.2024.07.004
- Primary Citation of Related Structures:
8RCD, 8RCF - PubMed Abstract:
Abasic sites are DNA lesions repaired by base excision repair. Cleavage of unrepaired abasic sites in single-stranded DNA (ssDNA) can lead to chromosomal breakage during DNA replication. How rupture of abasic DNA is prevented remains poorly understood. Here, using cryoelectron microscopy (cryo-EM), Xenopus laevis egg extracts, and human cells, we show that RAD51 nucleofilaments specifically recognize and protect abasic sites, which increase RAD51 association rate to DNA. In the absence of BRCA2 or RAD51, abasic sites accumulate as a result of DNA base methylation, oxidation, and deamination, inducing abasic ssDNA gaps that make replicating DNA fibers sensitive to APE1. RAD51 assembled on abasic DNA prevents abasic site cleavage by the MRE11-RAD50 complex, suppressing replication fork breakage triggered by an excess of abasic sites or POLθ polymerase inhibition. Our study highlights the critical role of BRCA2 and RAD51 in safeguarding against unrepaired abasic sites in DNA templates stemming from base alterations, ensuring genomic stability.
- IFOM, The AIRC Institute of Molecular Oncology, Milan, Italy.
Organizational Affiliation: