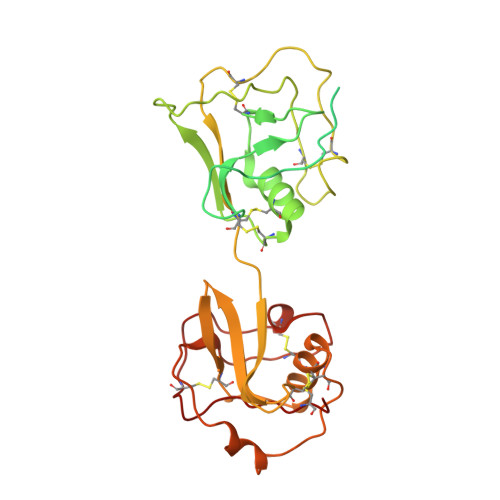
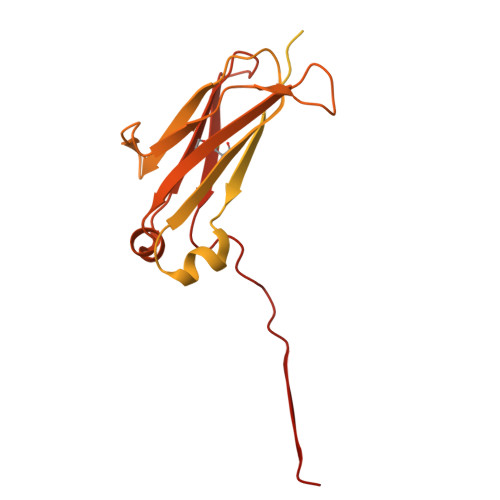
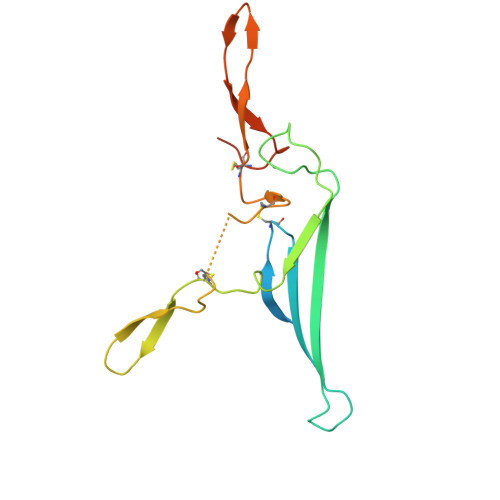
Cryo-EM reveals structural basis for human AIM/CD5L recognition of polymeric immunoglobulin M.
Chen, Q., Ishii, K., Mori, H., Nishijima, A., Arai, S., Miyazaki, T., Rosenthal, P.B.(2024) Nat Commun 15: 9387-9387
- PubMed: 39477921
- DOI: https://doi.org/10.1038/s41467-024-53615-5
- Primary Citation of Related Structures:
8R83, 8R84 - PubMed Abstract:
Cell surface scavenger receptors contribute to homoeostasis and the response to pathogens and products associated with damage by binding to common molecular features on a wide range of targets. Apoptosis inhibitor of macrophage (AIM/CD5L) is a soluble protein belonging to the scavenger receptor cysteine-rich (SRCR) superfamily that contributes to prevention of a wide range of diseases associated with infection, inflammation, and cancer. AIM forms complexes with IgM pentamers which helps maintain high-levels of circulating AIM in serum for subsequent activation on release from the complex. The structural basis for AIM recognition of IgM as well as other binding targets is unknown. Here we apply cryogenic electron microscopy imaging (cryo-EM) to show how interfaces on both of AIM's C-terminal SRCR domains interact with the Fcμ constant region and J chain components of the IgM core. Both SRCR interfaces are also shown to contribute interactions important for AIM binding to damage-associated molecular patterns (DAMPs).
- Structural Biology Science Technology Platform, The Francis Crick Institute, London, UK.
Organizational Affiliation: