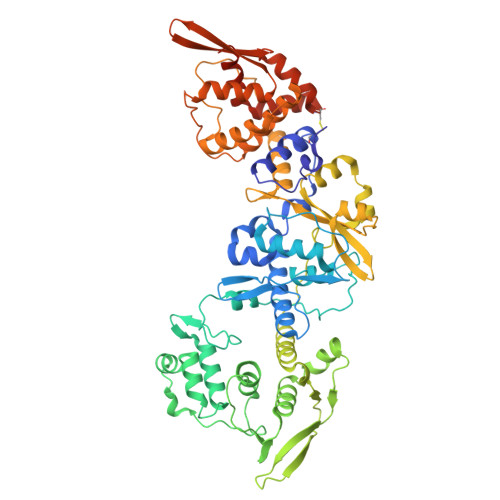
Palisade structure in intact vaccinia virions.
Hernandez-Gonzalez, M., Calcraft, T., Nans, A., Rosenthal, P.B., Way, M.(2024) mBio 15: e0313423-e0313423
- PubMed: 38171004
- DOI: https://doi.org/10.1128/mbio.03134-23
- Primary Citation of Related Structures:
8R5I - PubMed Abstract:
Vaccinia virus assembly in the cytoplasm of infected cells involves the formation of a biconcave viral core inside the maturing viral particle. The boundary of the core is defined by a pseudohexagonal palisade layer, composed of trimers projecting from an inner wall. To understand the assembly of this complex core architecture, we obtained a subnanometer structure of the palisade trimer by cryo-electron tomography and subtomogram averaging of purified intact virions. Using AlphaFold2 structure predictions, we determined that the palisade is formed from trimers of the proteolytically processed form of the viral protein A10. In addition, we found that each A10 protomer associates with an α-helix (residues 24-66) of A4. Cellular localization assays outside the context of infection demonstrate that the A4 N-terminus is necessary and sufficient to interact with A10. The interaction between A4 and A10 provides insights into how the palisade layer might become tightly associated with the viral membrane during virion maturation. Reconstruction of the palisade layer reveals that, despite local hexagonal ordering, the A10/A4 trimers are widely spaced, suggesting that additional components organize the lattice. This spacing would, however, allow the adoption of the characteristic biconcave shape of the viral core. Finally, we also found that the palisade incorporates multiple copies of a hexameric portal structure. We suggest that these portals are formed by E6, a viral protein that is essential for virion assembly and required to release viral mRNA from the core early in infection.IMPORTANCEPoxviruses such as variola virus (smallpox) and monkeypox cause diseases in humans. Other poxviruses, including vaccinia and modified vaccinia Ankara, are used as vaccine vectors. Given their importance, a greater structural understanding of poxvirus virions is needed. We now performed cryo-electron tomography of purified intact vaccinia virions to study the structure of the palisade, a protein lattice that defines the viral core boundary. We identified the main viral proteins that form the palisade and their interaction surfaces and provided new insights into the organization of the viral core.
- Cellular Signalling and Cytoskeletal Function Laboratory, The Francis Crick Institute, London, United Kingdom.
Organizational Affiliation: