Discovery of the sEH Inhibitor Epoxykynin as a Potent Kynurenine Pathway Modulator.
Dotsch, L., Davies, C., Hennes, E., Schonfeld, J., Kumar, A., Guita, C.D.C.L., Ehrler, J.H.M., Hiesinger, K., Thavam, S., Janning, P., Sievers, S., Knapp, S., Proschak, E., Ziegler, S., Waldmann, H.(2024) J Med Chem 67: 4691-4706
- PubMed: 38470246
- DOI: https://doi.org/10.1021/acs.jmedchem.3c02245
- Primary Citation of Related Structures:
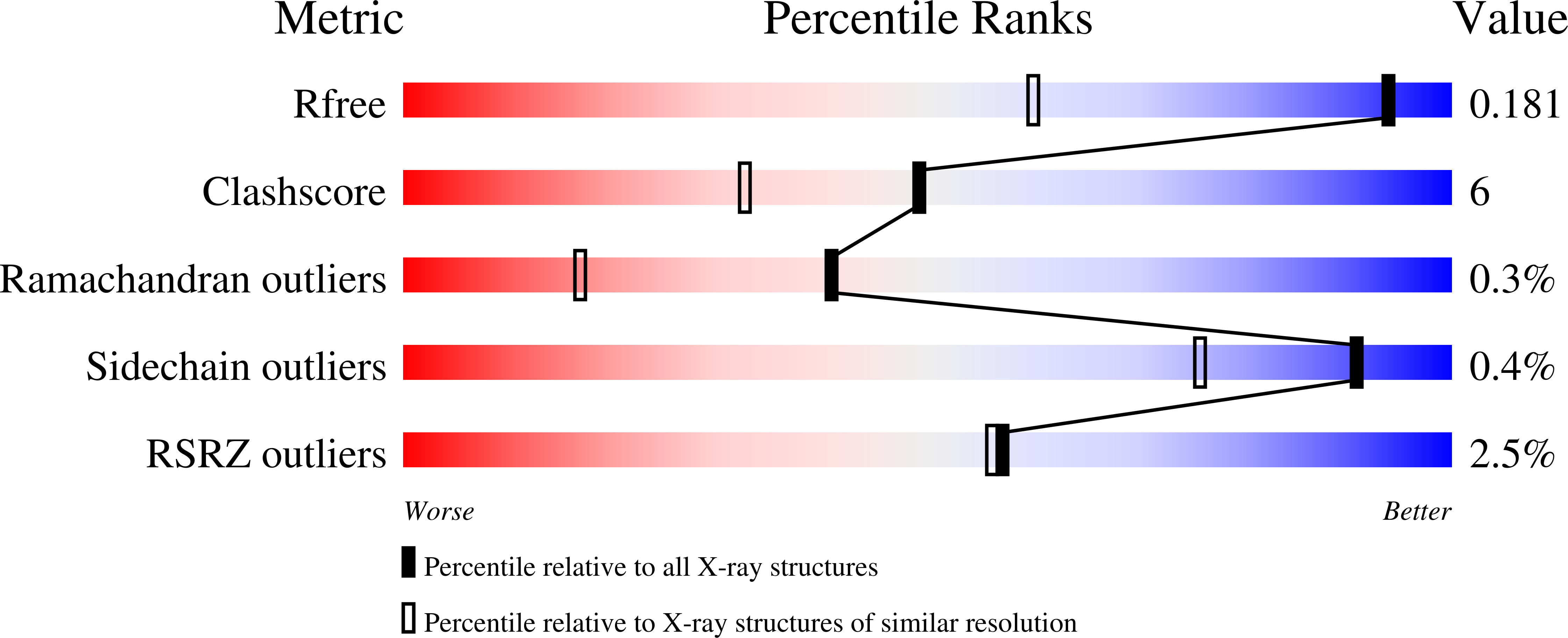
8QZD - PubMed Abstract:
Disease-related phenotypic assays enable unbiased discovery of novel bioactive small molecules and may provide novel insights into physiological systems and unprecedented molecular modes of action (MMOA). Herein, we report the identification and characterization of epoxykynin, a potent inhibitor of the soluble epoxide hydrolase (sEH). Epoxykynin was discovered by means of a cellular assay monitoring modulation of kynurenine (Kyn) levels in BxPC-3 cells upon stimulation with the cytokine interferon-γ (IFN-γ) and subsequent target identification employing affinity-based chemical proteomics. Increased Kyn levels are associated with immune suppression in the tumor microenvironment and, thus, the Kyn pathway and its key player indoleamine 2,3-dioxygenase 1 (IDO1) are appealing targets in immuno-oncology. However, targeting IDO1 directly has led to limited success in clinical investigations, demonstrating that alternative approaches to reduce Kyn levels are in high demand. We uncover a cross-talk between sEH and the Kyn pathway that may provide new opportunities to revert cancer-induced immune tolerance.
Organizational Affiliation:
Department of Chemical Biology, Max Planck Institute of Molecular Physiology, Otto-Hahn-Strasse 11, Dortmund 44227, Germany.