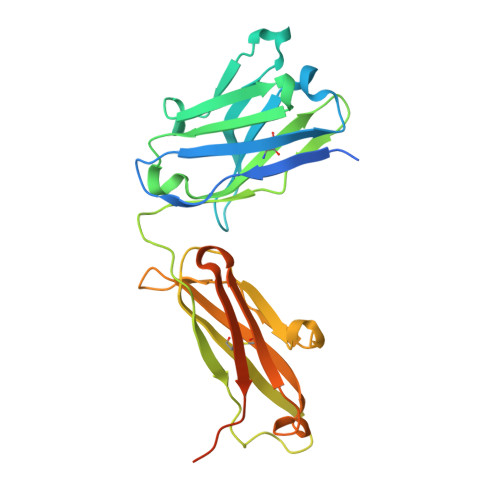
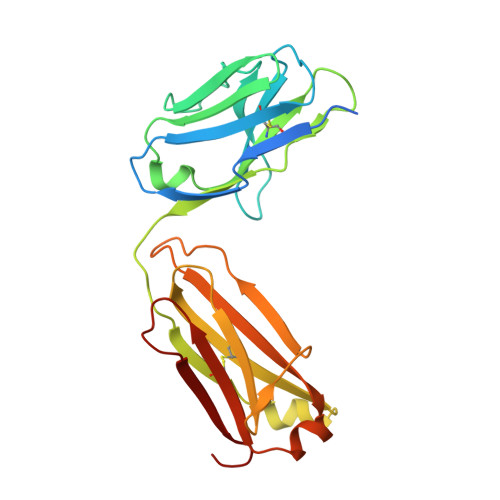
Covalent penicillin-protein conjugates elicit anti-drug antibodies that are clonally and functionally restricted.
Deimel, L.P., Moynie, L., Sun, G., Lewis, V., Turner, A., Buchanan, C.J., Burnap, S.A., Kutuzov, M., Kobras, C.M., Demyaneko, Y., Mohammed, S., Stracy, M., Struwe, W.B., Baldwin, A.J., Naismith, J., Davis, B.G., Sattentau, Q.J.(2024) Nat Commun 15: 6851-6851
- PubMed: 39127707
- DOI: https://doi.org/10.1038/s41467-024-51138-7
- Primary Citation of Related Structures:
8QXC - PubMed Abstract:
Many archetypal and emerging classes of small-molecule therapeutics form covalent protein adducts. In vivo, both the resulting conjugates and their off-target side-conjugates have the potential to elicit antibodies, with implications for allergy and drug sequestration. Although β-lactam antibiotics are a drug class long associated with these immunological phenomena, the molecular underpinnings of off-target drug-protein conjugation and consequent drug-specific immune responses remain incomplete. Here, using the classical β-lactam penicillin G (PenG), we probe the B and T cell determinants of drug-specific IgG responses to such conjugates in mice. Deep B cell clonotyping reveals a dominant murine clonal antibody class encompassing phylogenetically-related IGHV1, IGHV5 and IGHV10 subgroup gene segments. Protein NMR and x-ray structural analyses reveal that these drive structurally convergent binding modes in adduct-specific antibody clones. Their common primary recognition mechanisms of the penicillin side-chain moiety (phenylacetamide in PenG)-regardless of CDRH3 length-limits cross-reactivity against other β-lactam antibiotics. This immunogenetics-guided discovery of the limited binding solutions available to antibodies against side products of an archetypal covalent inhibitor now suggests future potential strategies for the 'germline-guided reverse engineering' of such drugs away from unwanted immune responses.
Organizational Affiliation:
Sir William Dunn School of Pathology, University of Oxford, Oxford, OX1 3RE, UK. Lachlan.Deimel@path.ox.ac.uk.