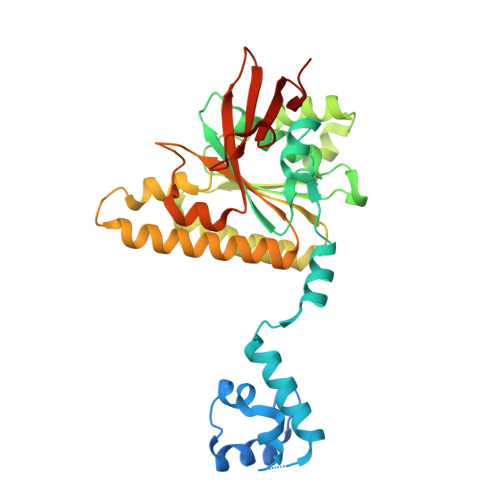
DMC1 and RAD51 bind FxxA and FxPP motifs of BRCA2 via two separate interfaces.
Miron, S., Legrand, P., Dupaigne, P., van Rossum-Fikkert, S.E., Ristic, D., Majeed, A., Kanaar, R., Zinn-Justin, S., Zelensky, A.N.(2024) Nucleic Acids Res
- PubMed: 38828772
- DOI: https://doi.org/10.1093/nar/gkae452
- Primary Citation of Related Structures:
8QQE - PubMed Abstract:
In vertebrates, the BRCA2 protein is essential for meiotic and somatic homologous recombination due to its interaction with the RAD51 and DMC1 recombinases through FxxA and FxPP motifs (here named A- and P-motifs, respectively). The A-motifs present in the eight BRC repeats of BRCA2 compete with the A-motif of RAD51, which is responsible for its self-oligomerization. BRCs thus disrupt RAD51 nucleoprotein filaments in vitro. The role of the P-motifs is less studied. We recently found that deletion of Brca2 exons 12-14 encoding one of them (the prototypical 'PhePP' motif), disrupts DMC1 but not RAD51 function in mouse meiosis. Here we provide a mechanistic explanation for this phenotype by solving the crystal structure of the complex between a BRCA2 fragment containing the PhePP motif and DMC1. Our structure reveals that, despite sharing a conserved phenylalanine, the A- and P-motifs bind to distinct sites on the ATPase domain of the recombinases. The P-motif interacts with a site that is accessible in DMC1 octamers and nucleoprotein filaments. Moreover, we show that this interaction also involves the adjacent protomer and thus increases the stability of the DMC1 nucleoprotein filaments. We extend our analysis to other P-motifs from RAD51AP1 and FIGNL1.
Organizational Affiliation:
Institute for Integrative Biology of the Cell (I2BC), Université Paris-Saclay, CEA, CNRS, Gif-sur-Yvette, France.