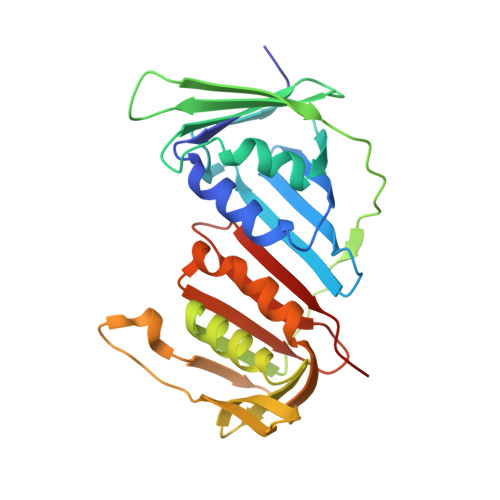
Canonical binding of Chaetomium thermophilum DNA polymerase delta / zeta subunit PolD3 and flap endonuclease Fen1 to PCNA.
Alphey, M.S., Wolford, C.B., MacNeill, S.A.(2023) Front Mol Biosci 10: 1320648-1320648
- PubMed: 38223238
- DOI: https://doi.org/10.3389/fmolb.2023.1320648
- Primary Citation of Related Structures:
8P9O, 8Q7I - PubMed Abstract:
The sliding clamp PCNA is a key player in eukaryotic genome replication and stability, acting as a platform onto which components of the DNA replication and repair machinery are assembled. Interactions with PCNA are frequently mediated via a short protein sequence motif known as the PCNA-interacting protein (PIP) motif. Here we describe the binding mode of a PIP motif peptide derived from C-terminus of the PolD3 protein from the thermophilic ascomycete fungus C. thermophilum , a subunit of both DNA polymerase δ (Pol δ) and the translesion DNA synthesis polymerase Pol ζ, characterised by isothermal titration calorimetry (ITC) and protein X-ray crystallography. In sharp contrast to the previously determined structure of a Chaetomium thermophilum PolD4 peptide bound to PCNA, binding of the PolD3 peptide is strictly canonical, with the peptide adopting the anticipated 3 10 helix structure, conserved Gln441 inserting into the so-called Q-pocket on PCNA, and Ile444 and Phe448 forming a two-fork plug that inserts into the hydrophobic surface pocket on PCNA. The binding affinity for the canonical PolD3 PIP-PCNA interaction determined by ITC is broadly similar to that previously determined for the non-canonical PolD4 PIP-PCNA interaction. In addition, we report the structure of a PIP peptide derived from the C. thermophilum Fen1 nuclease bound to PCNA. Like PolD3, Fen1 PIP peptide binding to PCNA is achieved by strictly canonical means. Taken together, these results add to an increasing body of information on how different proteins bind to PCNA, both within and across species.
Organizational Affiliation:
Biomedical Sciences Research Complex, School of Biology, University of St Andrews, St Andrews, United Kingdom.