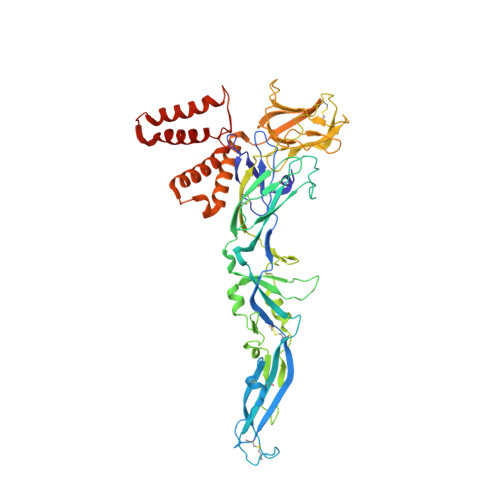
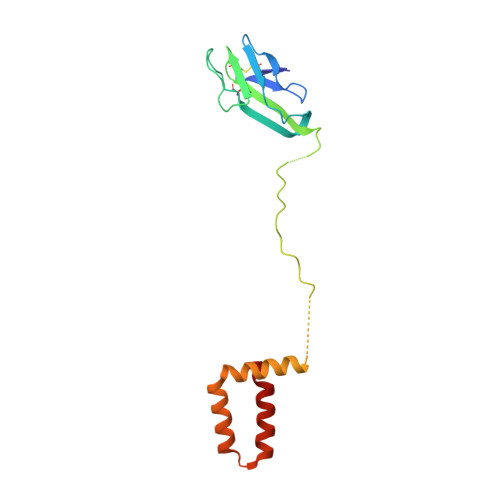
The structure of immature tick-borne encephalitis virus supports the collapse model of flavivirus maturation.
Anastasina, M., Fuzik, T., Domanska, A., Pulkkinen, L.I.A., Smerdova, L., Formanova, P.P., Strakova, P., Novacek, J., Ruzek, D., Plevka, P., Butcher, S.J.(2024) Sci Adv 10: eadl1888-eadl1888
- PubMed: 38959313
- DOI: https://doi.org/10.1126/sciadv.adl1888
- Primary Citation of Related Structures:
8PPQ, 8PUV - PubMed Abstract:
We present structures of three immature tick-borne encephalitis virus (TBEV) isolates. Our atomic models of the major viral components, the E and prM proteins, indicate that the pr domains of prM have a critical role in holding the heterohexameric prM3E3 spikes in a metastable conformation. Destabilization of the prM furin-sensitive loop at acidic pH facilitates its processing. The prM topology and domain assignment in TBEV is similar to the mosquito-borne Binjari virus, but is in contrast to other immature flavivirus models. These results support that prM cleavage, the collapse of E protein ectodomains onto the virion surface, the large movement of the membrane domains of both E and M, and the release of the pr fragment from the particle render the virus mature and infectious. Our work favors the collapse model of flavivirus maturation warranting further studies of immature flaviviruses to determine the sequence of events and mechanistic details driving flavivirus maturation.
- Faculty of Biological and Environmental Sciences, Molecular and Integrative Bioscience Research Programme, University of Helsinki, Helsinki, Finland.
Organizational Affiliation: