Fragment-based assembly of a symmetric protein scaffold
Wouters, S.M.L., Noguchi, H., Voet, A.R.D.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Kelch-like ECH-associated protein 1 | 143 | Homo sapiens | Mutation(s): 0 Gene Names: KEAP1, INRF2, KIAA0132, KLHL19 | 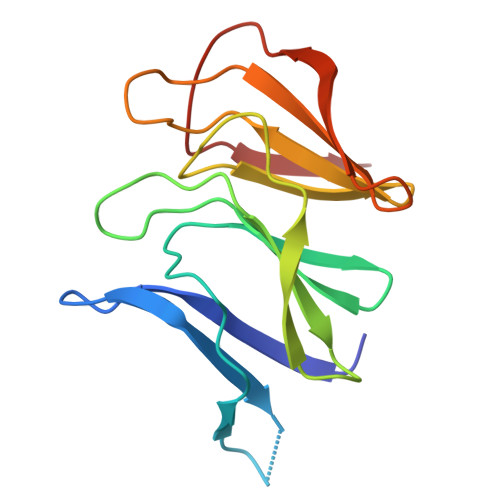 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q14145 (Homo sapiens) Explore Q14145 Go to UniProtKB: Q14145 | |||||
PHAROS: Q14145 GTEx: ENSG00000079999 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q14145 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 46.846 | α = 90 |
| b = 220.962 | β = 90 |
| c = 248.75 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| Aimless | data scaling |
| XDS | data reduction |
| MOLREP | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Research Foundation - Flanders (FWO) | Belgium | 1S89918N |
| Research Foundation - Flanders (FWO) | Belgium | G0F9316N |
| Research Foundation - Flanders (FWO) | Belgium | G051917N |