Computational design of the SAKe scaffold proteins
Wouters, S.M.L., Noguchi, H., Voet, A.R.D.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| SAKe6EEref | 298 | synthetic construct | Mutation(s): 0 | 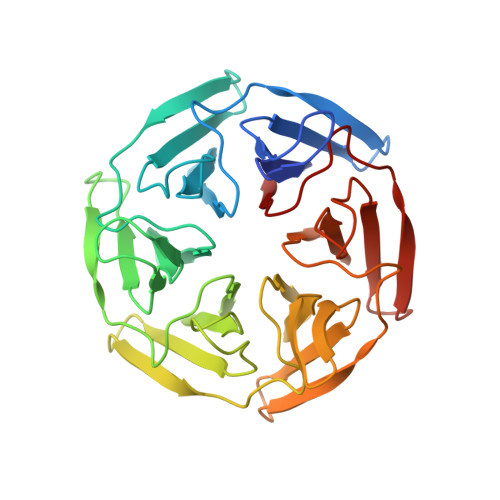 | |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 47.048 | α = 90 |
| b = 66.661 | β = 117.59 |
| c = 48.243 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| Aimless | data scaling |
| XDS | data reduction |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Research Foundation - Flanders (FWO) | Belgium | 1S89918N |
| Research Foundation - Flanders (FWO) | Belgium | G0F9316N |
| Research Foundation - Flanders (FWO) | Belgium | G051917N |