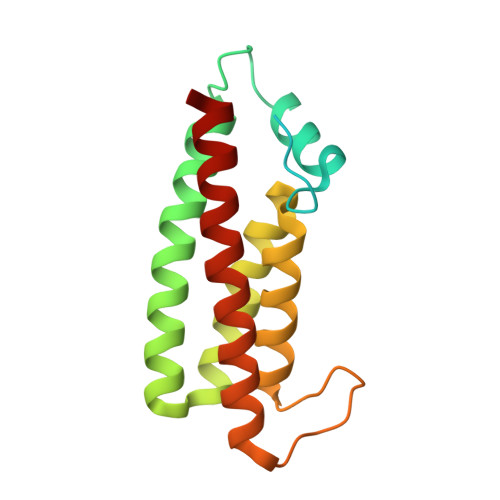
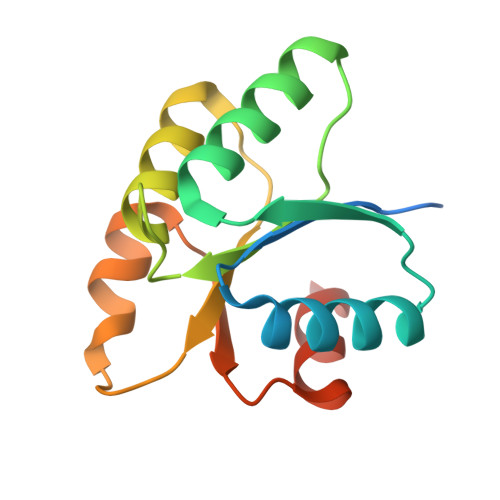
Structural and functional insights underlying recognition of histidine phosphotransfer protein in fungal phosphorelay systems.
Paredes-Martinez, F., Eixeres, L., Zamora-Caballero, S., Casino, P.(2024) Commun Biol 7: 814-814
- PubMed: 38965424
- DOI: https://doi.org/10.1038/s42003-024-06459-0
- Primary Citation of Related Structures:
8PBW, 8PDC, 8PHN, 8PHX, 8RQG, 8RQJ - PubMed Abstract:
In human pathogenic fungi, receiver domains from hybrid histidine kinases (hHK) have to recognize one HPt. To understand the recognition mechanism, we have assessed phosphorelay from receiver domains of five hHKs of group III, IV, V, VI, and XI to HPt from Chaetomium thermophilum and obtained the structures of Ct_HPt alone and in complex with the receiver domain of hHK group VI. Our data indicate that receiver domains phosphotransfer to Ct_HPt, show a low affinity for complex formation, and prevent a Leu-Thr switch to stabilize phosphoryl groups, also derived from the structures of the receiver domains of hHK group III and Candida albicans Sln1. Moreover, we have elucidated the envelope structure of C. albicans Ypd1 using small-angle X-ray scattering which reveals an extended flexible conformation of the long loop αD-αE which is not involved in phosphotransfer. Finally, we have analyzed the role of salt bridges in the structure of Ct_HPt alone.
- Departamento de Bioquímica y Biología Molecular, Universitat de València, Burjassot, Spain.
Organizational Affiliation: